

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50176071 (2-(2-Amino-ethylamino)-N-(7-{2-[N'-(5-nitro-furan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase | J Med Chem 48: 7024-39 (2005) Article DOI: 10.1021/jm050256l BindingDB Entry DOI: 10.7270/Q2CN73F9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50121447 (5-Nitro-furan-2-carboxylic acid N'-(2-naphthalen-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase | J Med Chem 48: 7024-39 (2005) Article DOI: 10.1021/jm050256l BindingDB Entry DOI: 10.7270/Q2CN73F9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50352164 (CHEMBL1824793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 344 | n/a | n/a | n/a | n/a | n/a | n/a |
Batman University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glutathione reductase | Bioorg Med Chem Lett 21: 5398-402 (2011) Article DOI: 10.1016/j.bmcl.2011.07.002 BindingDB Entry DOI: 10.7270/Q2V69KKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096018 (6-(3-METHYL-1,4-DIOXO-1,4-DIHYDRONAPHTHALEN-2-YL)H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of Plasmodium falciparum Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096035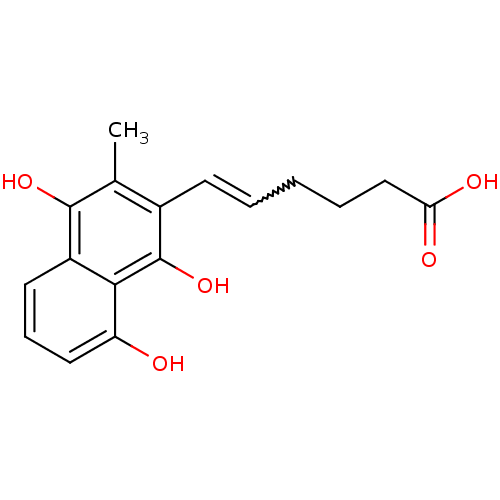 (6-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of human Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052014 (3-Methyl-10-(3-trifluoromethyl-phenyl)-10H-benzo[g...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052000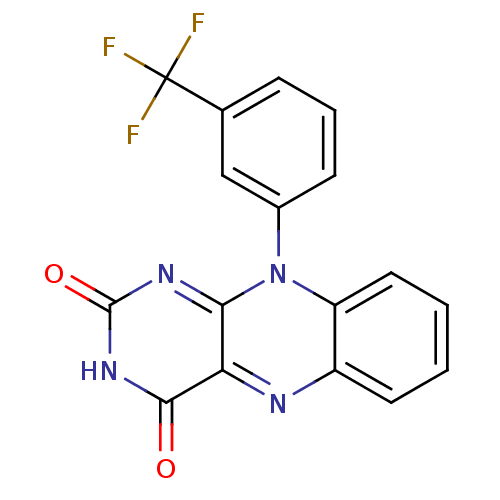 (10-(3-Trifluoromethyl-phenyl)-10H-benzo[g]pteridin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096062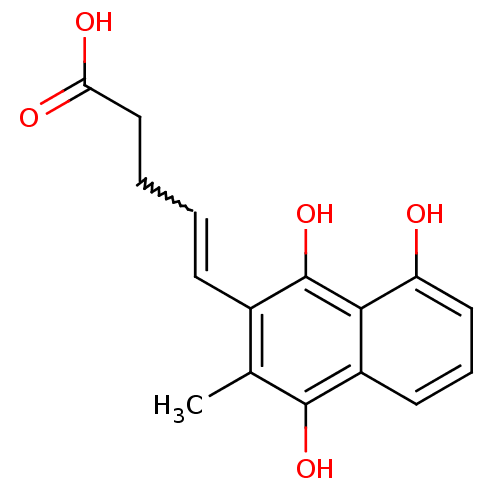 (5-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of human Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50051997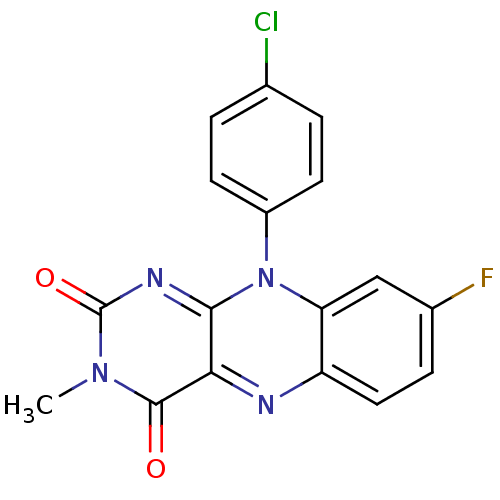 (10-(4-Chloro-phenyl)-8-fluoro-3-methyl-10H-benzo[g...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096035 (6-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of Plasmodium falciparum Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50051998 (3-Methyl-10-naphthalen-1-yl-10H-benzo[g]pteridine-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052004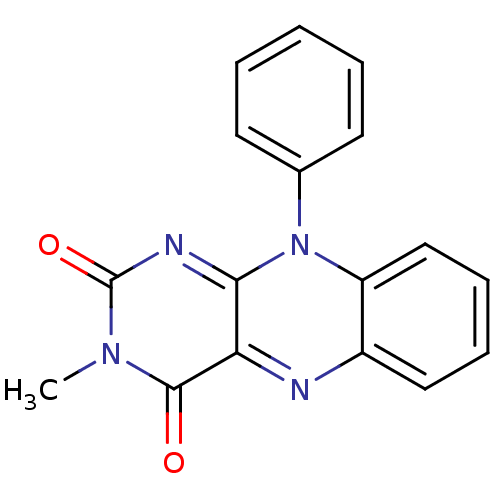 (3-Methyl-10-phenyl-10H-benzo[g]pteridine-2,4-dione...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052001 (8-Azido-10-(4-chloro-phenyl)-3-methyl-10H-benzo[g]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096062 (5-(8-Hydroxy-3-methyl-1,4-dioxo-1,4-dihydro-naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of Plasmodium falciparum Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096018 (6-(3-METHYL-1,4-DIOXO-1,4-DIHYDRONAPHTHALEN-2-YL)H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of human Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096018 (6-(3-METHYL-1,4-DIOXO-1,4-DIHYDRONAPHTHALEN-2-YL)H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase in the absence of glucose-6-phosphate dehydrogenase (G6PDH) | J Med Chem 47: 5972-83 (2004) Article DOI: 10.1021/jm0497545 BindingDB Entry DOI: 10.7270/Q20R9NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096018 (6-(3-METHYL-1,4-DIOXO-1,4-DIHYDRONAPHTHALEN-2-YL)H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase in the the presence of 200 uM exogenous NADP | J Med Chem 47: 5972-83 (2004) Article DOI: 10.1021/jm0497545 BindingDB Entry DOI: 10.7270/Q20R9NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096071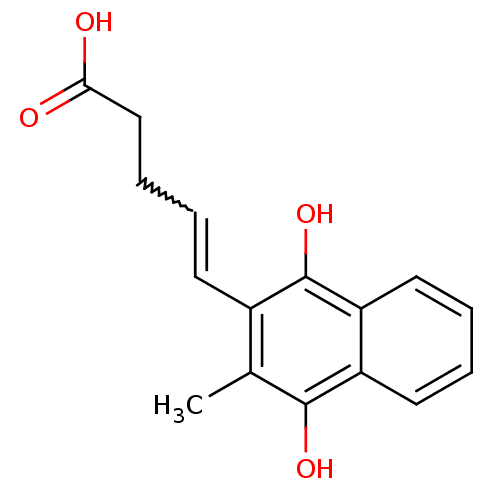 (5-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of human Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096018 (6-(3-METHYL-1,4-DIOXO-1,4-DIHYDRONAPHTHALEN-2-YL)H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase in the the presence of 200 uM exogenous NADP | J Med Chem 47: 5972-83 (2004) Article DOI: 10.1021/jm0497545 BindingDB Entry DOI: 10.7270/Q20R9NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50051996 (3-Methyl-10-pyridin-4-yl-10H-benzo[g]pteridine-2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096018 (6-(3-METHYL-1,4-DIOXO-1,4-DIHYDRONAPHTHALEN-2-YL)H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibitory concentration against human glutathione reductase | J Med Chem 47: 5972-83 (2004) Article DOI: 10.1021/jm0497545 BindingDB Entry DOI: 10.7270/Q20R9NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052006 (10-Pentafluorophenyl-10H-benzo[g]pteridine-2,4-dio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052007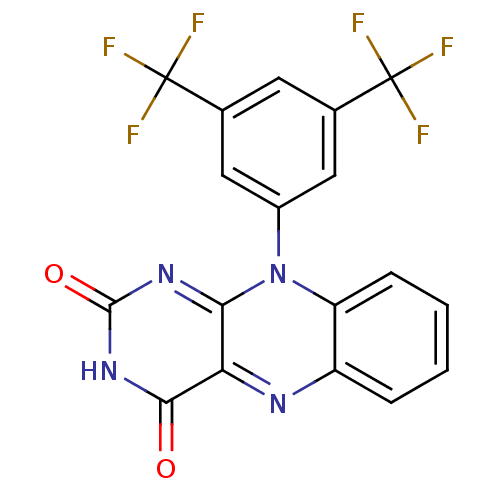 (10-(3,5-Bis-trifluoromethyl-phenyl)-10H-benzo[g]pt...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50051994 (10-Anthracen-1-yl-3-methyl-10H-benzo[g]pteridine-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50096071 (5-(3-Methyl-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 8525 CNRS-Université Lille II-Institut Pasteur de Lille Curated by ChEMBL | Assay Description In vitro inhibition of Plasmodium falciparum Glutathione Reductase | J Med Chem 44: 4268-76 (2001) BindingDB Entry DOI: 10.7270/Q20V8C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052010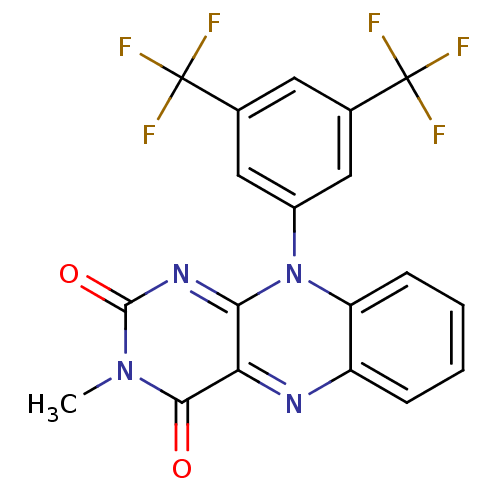 (10-(3,5-Bis-trifluoromethyl-phenyl)-3-methyl-10H-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50177103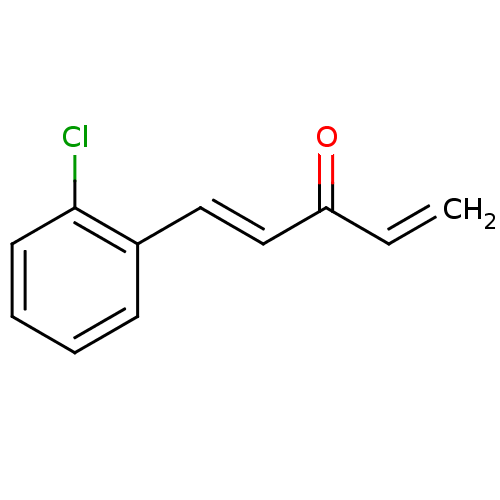 (1-(2'-chlorophenyl)penta-1,4-dien-3-one | CHEMBL22...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Biochemie-Zentrum der Universität Heidelberg Curated by ChEMBL | Assay Description Inhibition of human glutathione reductase after 5 mins preincubation with NADPH in presence of 500 uM GSSG substrate | J Med Chem 48: 7400-10 (2005) Article DOI: 10.1021/jm0504860 BindingDB Entry DOI: 10.7270/Q23X8662 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50316592 ((2R(S),7R(S))-7-Hydroxybicyclo[2.2.1]heptan-2-yl n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052013 (CHEMBL415870 | [10-(3,5-Dichloro-phenyl)-2,4-dioxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293668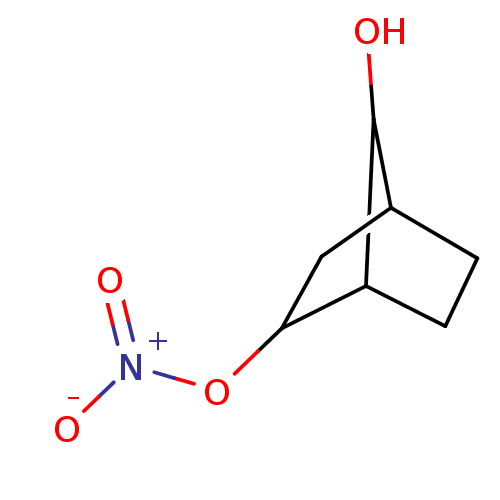 ((2S(R),7R(S))-7-Hydroxybicyclo[2.2.1]heptan-2-yl n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293667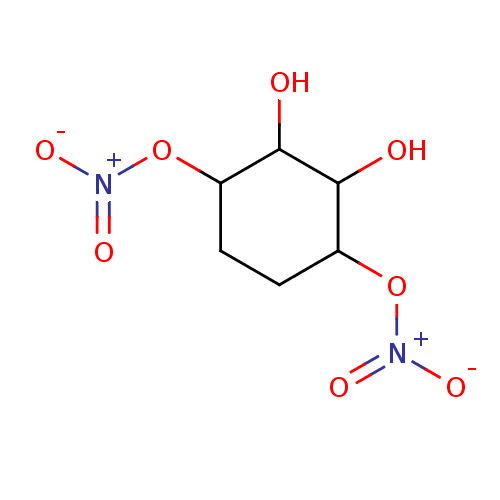 ((1R(S),2R(S),3S(R),4S(R))-2,3-Dihydroxycyclo-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50339129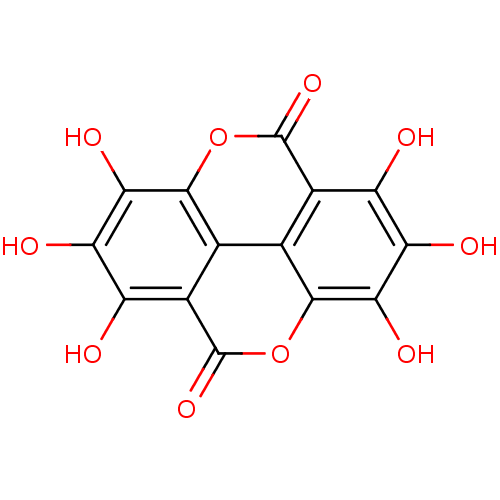 (CHEMBL1688544 | Coruleoellagic acid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Justus-Liebig-University Curated by ChEMBL | Assay Description Inhibition of human glutathione reductase by spectrophotometer | Antimicrob Agents Chemother 53: 622-30 (2009) Article DOI: 10.1128/AAC.00544-08 BindingDB Entry DOI: 10.7270/Q2RR1ZJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052011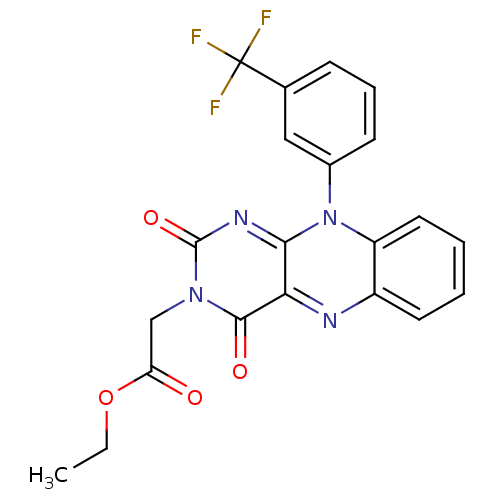 (CHEMBL38563 | [2,4-Dioxo-10-(3-trifluoromethyl-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052002 (3-Methyl-10-pyridin-3-yl-10H-benzo[g]pteridine-2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293666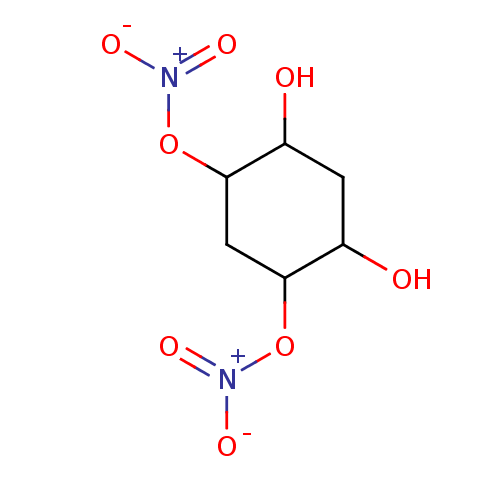 ((1S(R),3S(R),4S(R),6S(R))-4,6-Dihydroxycyclo-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50015950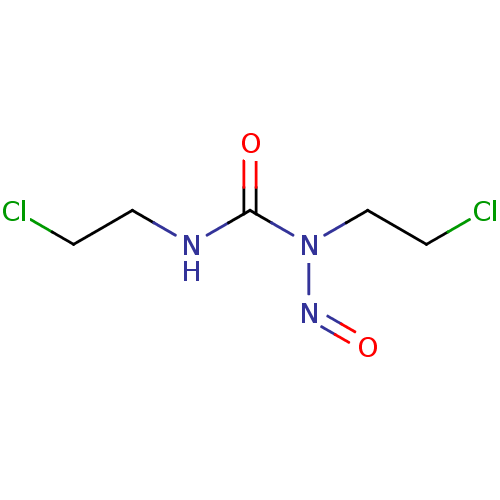 (1,3-bis(2-chloroethyl)-1-nitrosourea | Bicnu (TN) ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Minas Gerais Curated by ChEMBL | Assay Description Inhibition of human recombinant glutathione reductase using glutathione as substrate preincubated for 30 mins by colorimetric assay | Eur J Med Chem 71: 282-9 (2014) Article DOI: 10.1016/j.ejmech.2013.11.011 BindingDB Entry DOI: 10.7270/Q25Q4XKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293665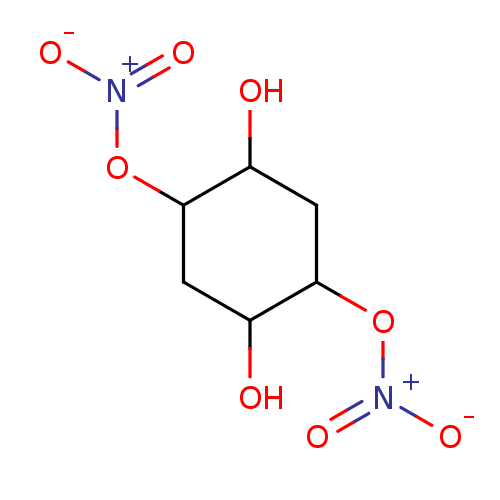 ((1R(S),2R(S),4R(S),5R(S))-2,5-Dihydroxycyclo-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM522150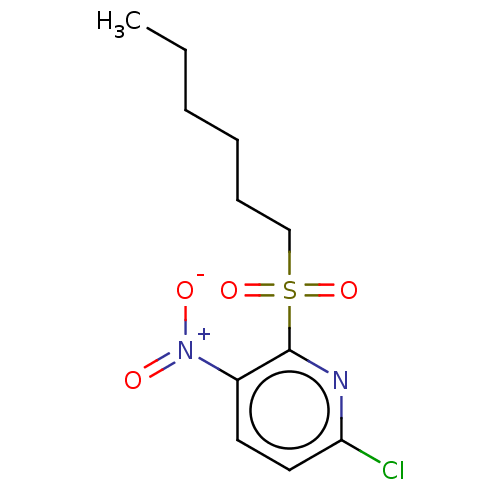 (US11161815, Example 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Small molecule inhibition of recombinant thioredoxin reductase 1 (TrxR1) and gluthathione reductase (GR) was examined in 96-well plate format. 30 nM ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2G73HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM501861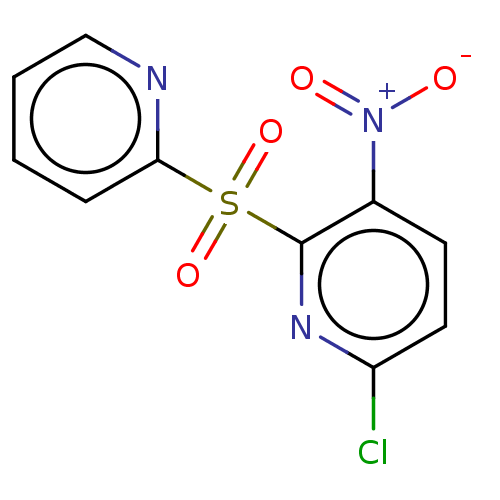 (US11028067, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Oblique Therapeutics AB US Patent | Assay Description Small molecule inhibition of recombinant thioredoxin reductase 1 (TrxR1) and gluthathione reductase (GR) was examined in 96-well plate format. 30 nM ... | US Patent US11028067 (2021) BindingDB Entry DOI: 10.7270/Q2Q81H65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50104661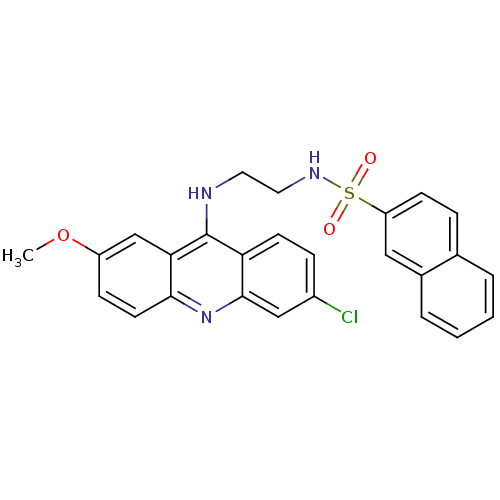 (CHEMBL92155 | Naphthalene-2-sulfonic acid [2-(6-ch...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against human Glutathione reductase was determined | Bioorg Med Chem Lett 11: 2655-7 (2001) BindingDB Entry DOI: 10.7270/Q2154G90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293664 (9(R(S))-Hydroxy-1,2,3,4-tetrahydro-1,4-methano-nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293663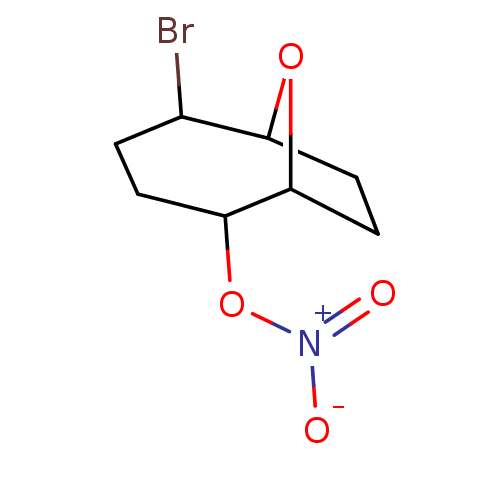 ((1S(R),2S(R),5R(S),6R(S))-5-Bromo-9-oxabicyclo[4.2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM501863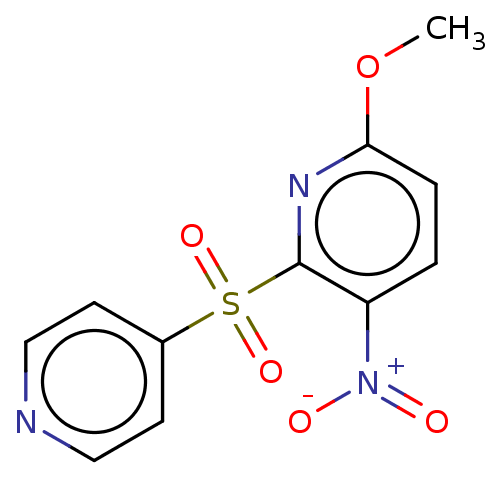 (US11028067, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Oblique Therapeutics AB US Patent | Assay Description Small molecule inhibition of recombinant thioredoxin reductase 1 (TrxR1) and gluthathione reductase (GR) was examined in 96-well plate format. 30 nM ... | US Patent US11028067 (2021) BindingDB Entry DOI: 10.7270/Q2Q81H65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM94597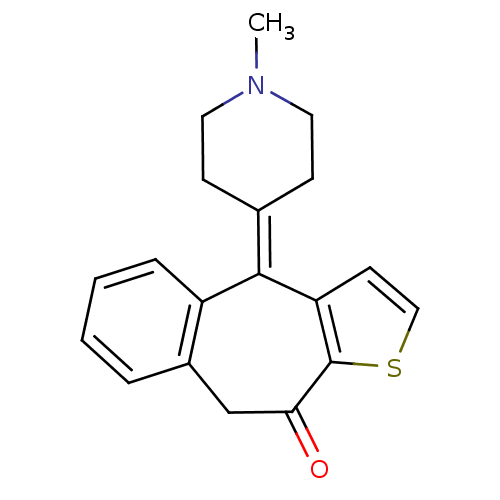 ((Z)-2-butenedioate;10-(1-methyl-4-piperidinylidene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 800 | -8.31 | 1.20E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Ataturk University | Assay Description GR activity was determined by the method of Carlberg and Mannervik [Carlberg et al., FL:Academic Press, 72:248-254] with a Shimadzu Spectrophotometer... | J Enzyme Inhib Med Chem 27: 18-23 (2012) Article DOI: 10.3109/14756366.2011.572879 BindingDB Entry DOI: 10.7270/Q20K27GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052005 (CHEMBL289205 | [10-(4-Chloro-phenyl)-2,4-dioxo-4,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50054332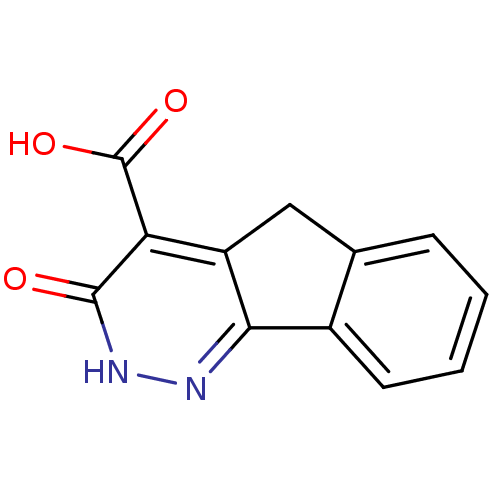 (3-Oxo-3,5-dihydro-2H-indeno[1,2-c]pyridazine-4-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glutathione reductase | J Med Chem 39: 4396-405 (1996) Article DOI: 10.1021/jm960124f BindingDB Entry DOI: 10.7270/Q2N58N21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50352163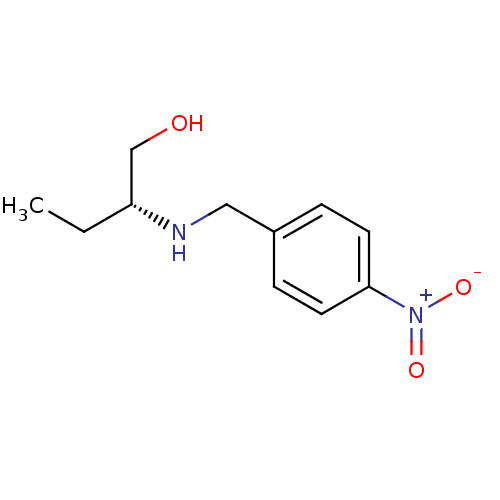 (CHEMBL1824792) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Batman University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glutathione reductase | Bioorg Med Chem Lett 21: 5398-402 (2011) Article DOI: 10.1016/j.bmcl.2011.07.002 BindingDB Entry DOI: 10.7270/Q2V69KKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50104665 (CHEMBL89368 | Naphthalene-2-sulfonic acid [4-(6-ch...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against human Glutathione reductase was determined | Bioorg Med Chem Lett 11: 2655-7 (2001) BindingDB Entry DOI: 10.7270/Q2154G90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50052008 (CHEMBL414176 | [10-(3,5-Dichloro-phenyl)-2,4-dioxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie II der Universität Curated by ChEMBL | Assay Description Inhibitory activity against human glutathione reductase in presence of 100 microM GSSG | J Med Chem 39: 1549-54 (1996) Article DOI: 10.1021/jm950511+ BindingDB Entry DOI: 10.7270/Q2F18XTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50293661 (CHEMBL556256 | trans-(1S(R),2S(R))-2-Hydroxycycloo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Atat£rk University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte glutathione reductase | Bioorg Med Chem Lett 19: 3661-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.087 BindingDB Entry DOI: 10.7270/Q2930V34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 203 total ) | Next | Last >> |