

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50408050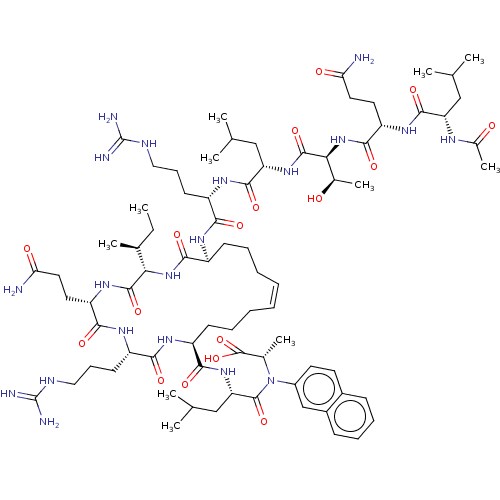 (CHEMBL5282944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396286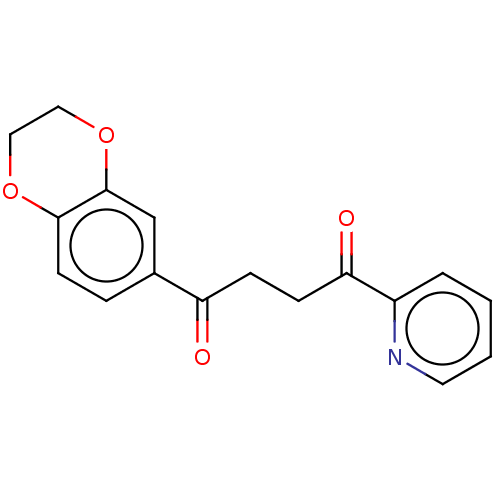 (US10314832, Compound 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396284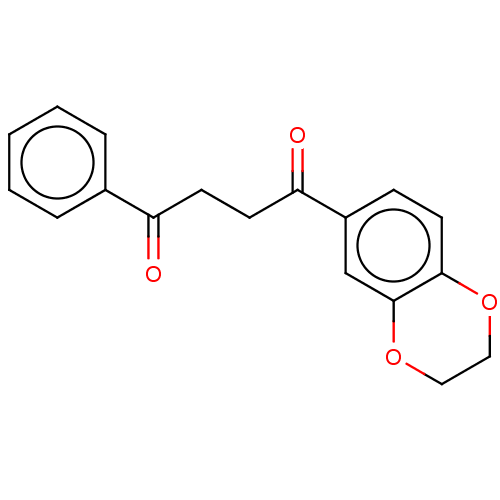 (US10314832, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 28.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396287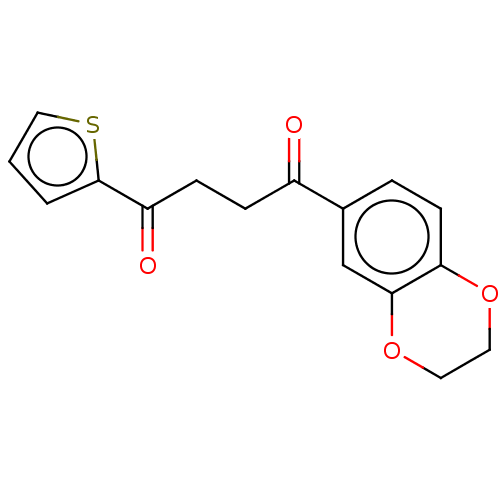 (US10314832, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396292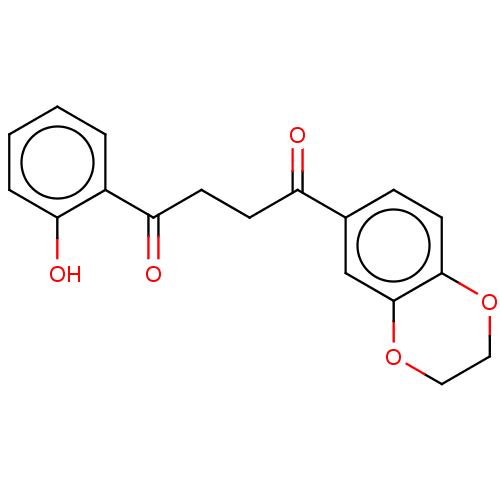 (US10314832, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396291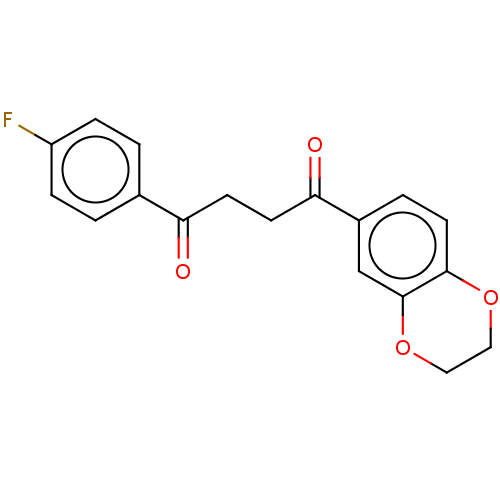 (US10314832, Compound 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50014132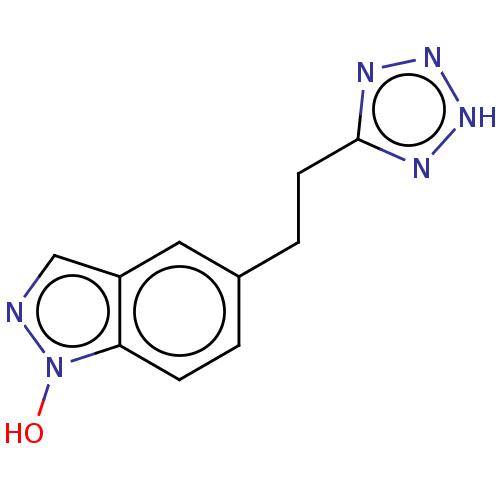 (CHEMBL2323032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396288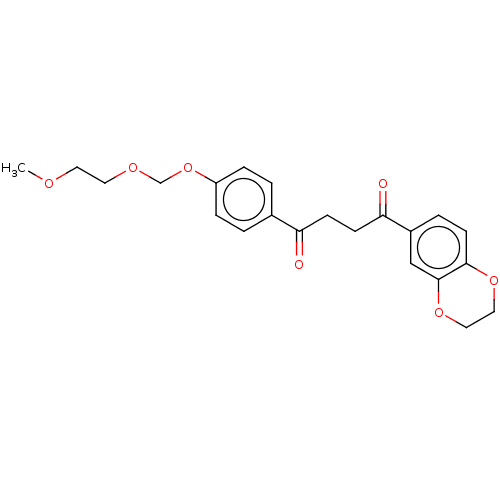 (US10314832, Compound 11 | US10314832, Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 805 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396289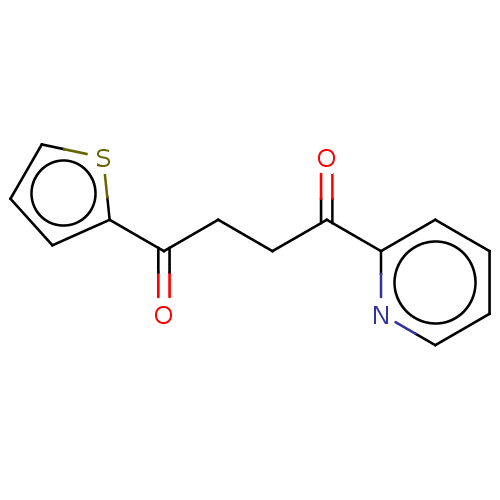 (US10314832, Compound 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396298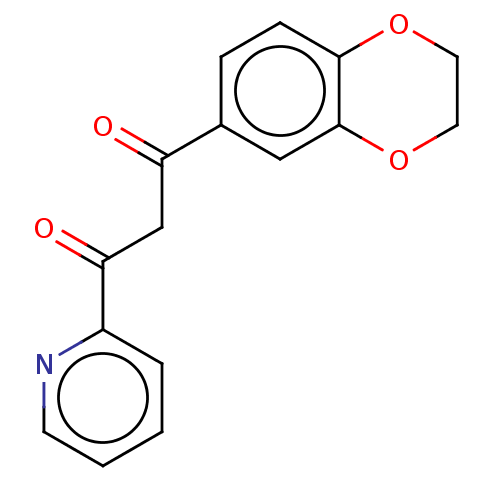 (US10314832, Compound 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509812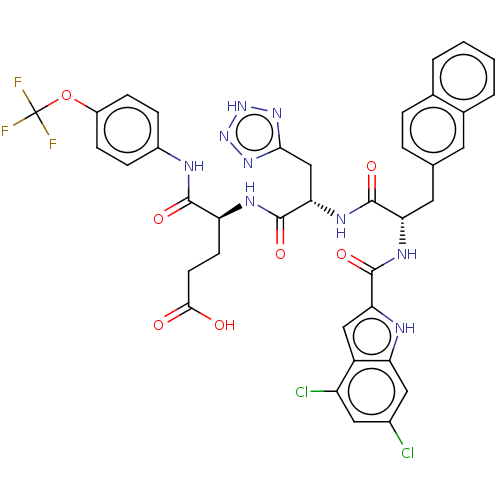 (CHEMBL4548532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50589223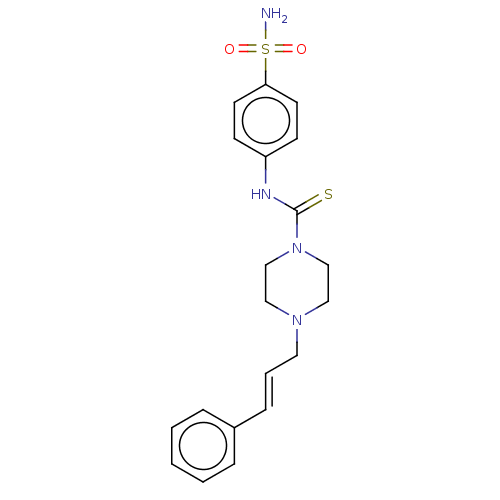 (CHEMBL5185515) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00228 BindingDB Entry DOI: 10.7270/Q2GQ72Q3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396290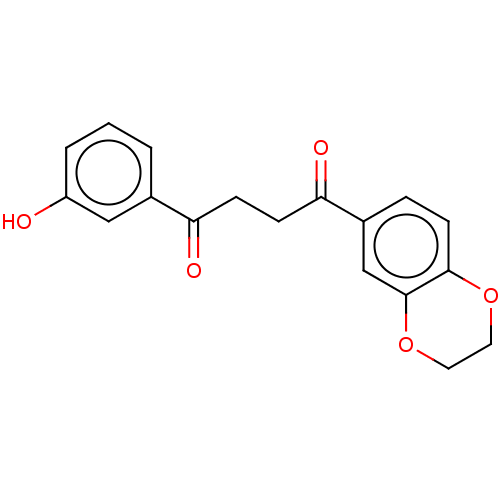 (US10314832, Compound 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509813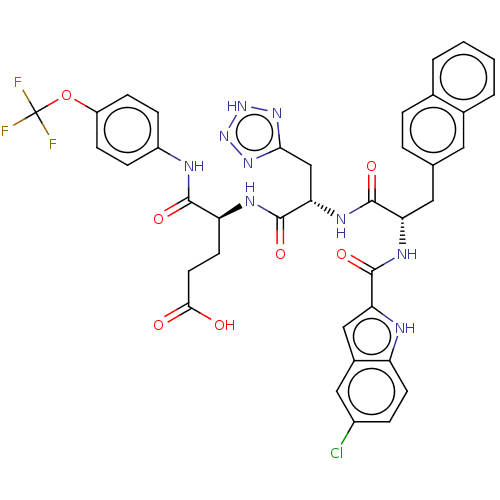 (CHEMBL4451478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396299 (US10314832, Compound 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396302 (US10314832, Compound 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50436055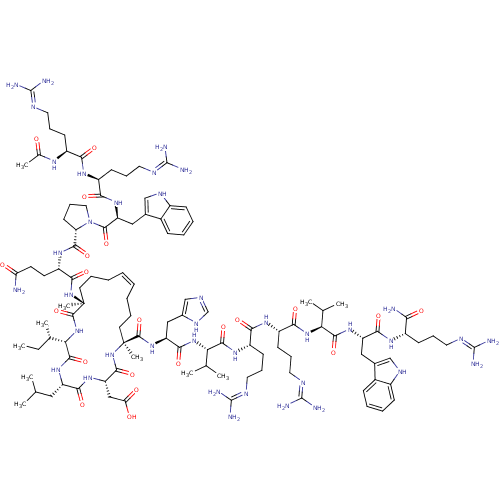 (CHEMBL2397076) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of beta-catenin-mediated transcriptional activity in Wnt3a-stimulated human HeLa cells after 24 hrs by Dual-Glo luciferase reporter gene a... | Bioorg Med Chem 21: 4020-6 (2013) Article DOI: 10.1016/j.bmc.2013.02.050 BindingDB Entry DOI: 10.7270/Q2BP046Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50436054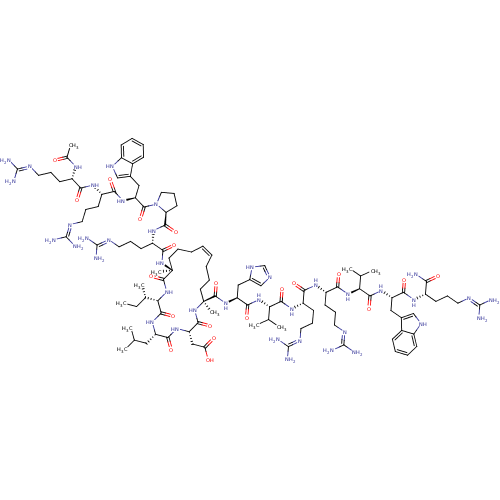 (CHEMBL2397077) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of beta-catenin-mediated transcriptional activity in Wnt3a-stimulated human HeLa cells after 24 hrs by Dual-Glo luciferase reporter gene a... | Bioorg Med Chem 21: 4020-6 (2013) Article DOI: 10.1016/j.bmc.2013.02.050 BindingDB Entry DOI: 10.7270/Q2BP046Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509810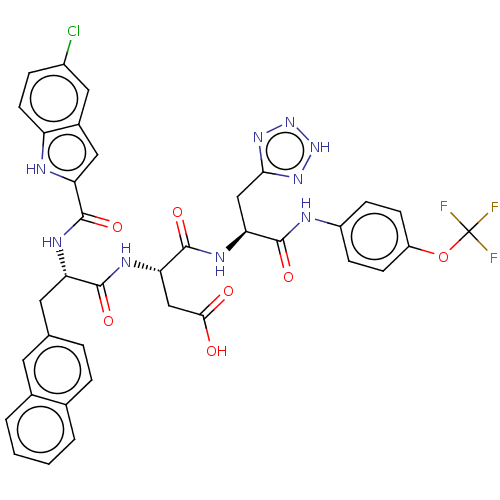 (CHEMBL4464142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396301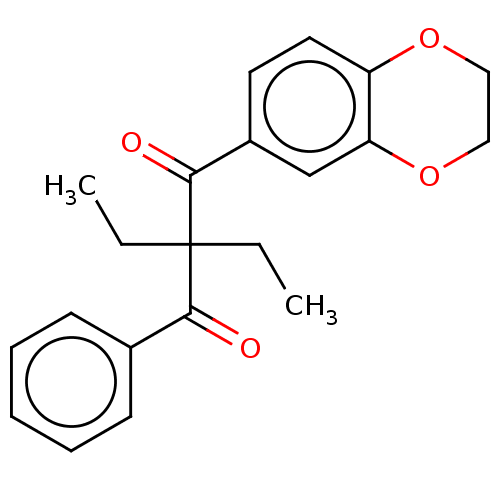 (US10314832, Compound 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50014148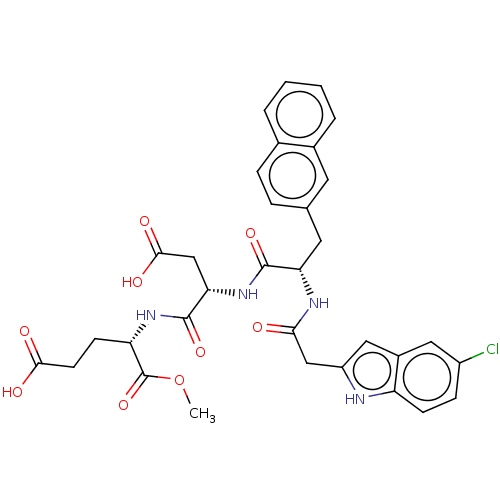 (CHEMBL3260853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of human wild-type beta-catenin (residues 138-686)/C-terminally fluorescein labeled human wild-type Tcf4 (residues 7-51) interaction after... | J Med Chem 58: 4678-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00223 BindingDB Entry DOI: 10.7270/Q2G44S28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509801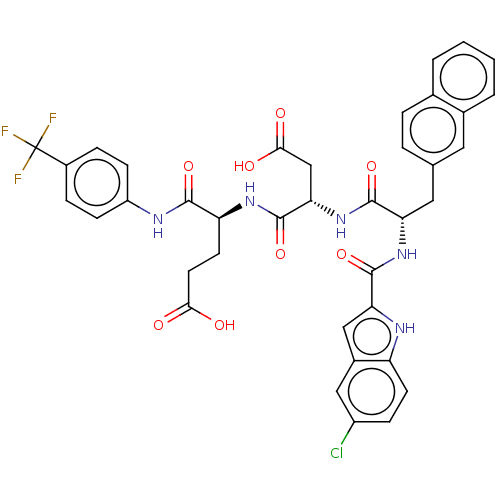 (CHEMBL4464433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509781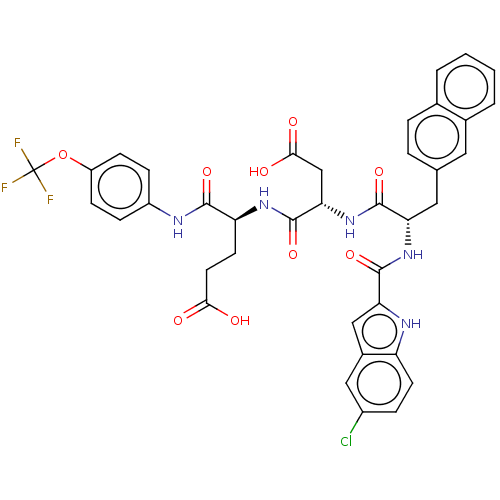 (CHEMBL4450852) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509803 (CHEMBL4449577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509802 (CHEMBL4515157) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509818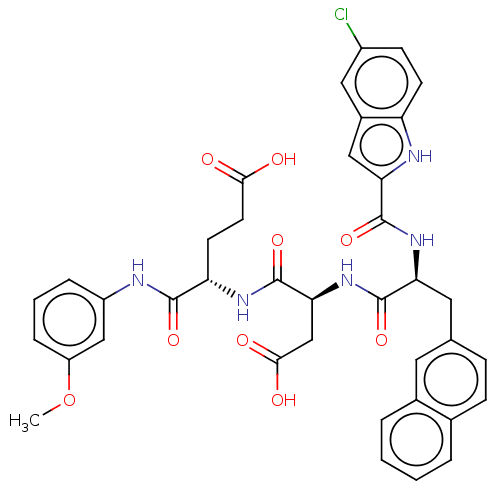 (CHEMBL4579221) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509819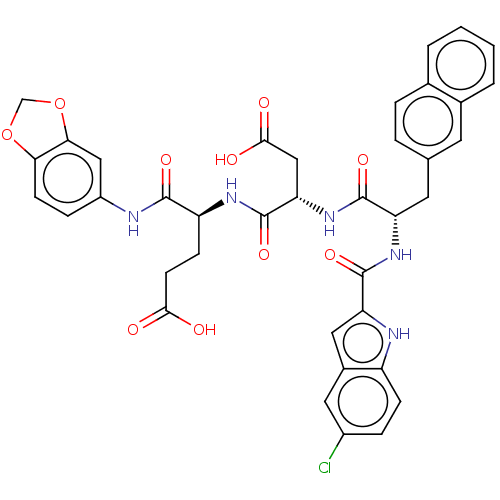 (CHEMBL4460073) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509804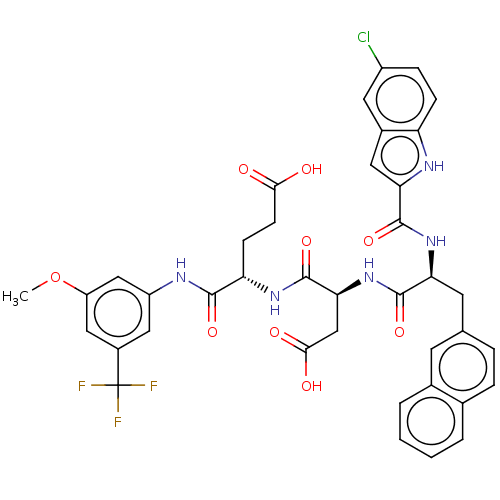 (CHEMBL4455488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396293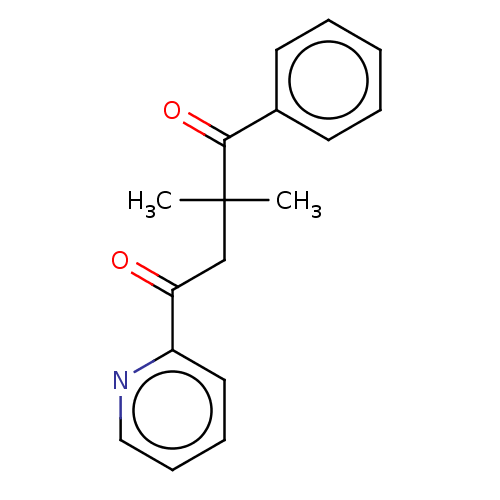 (US10314832, Compound 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396297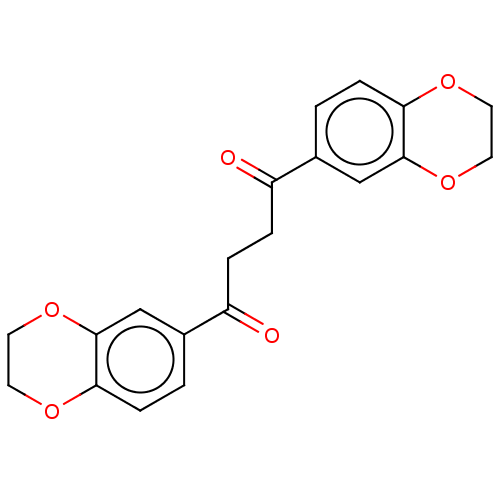 (US10314832, Compound 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396294 (US10314832, Compound 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396300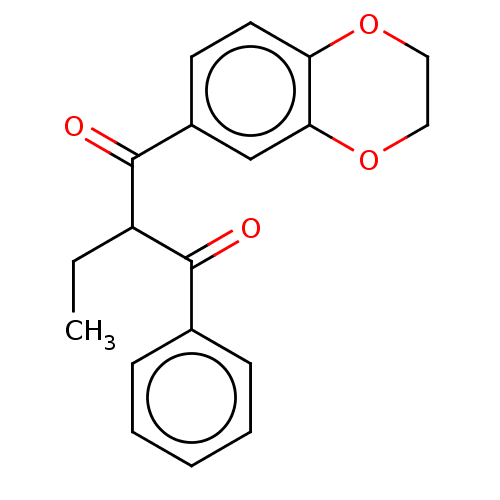 (US10314832, Compound 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509817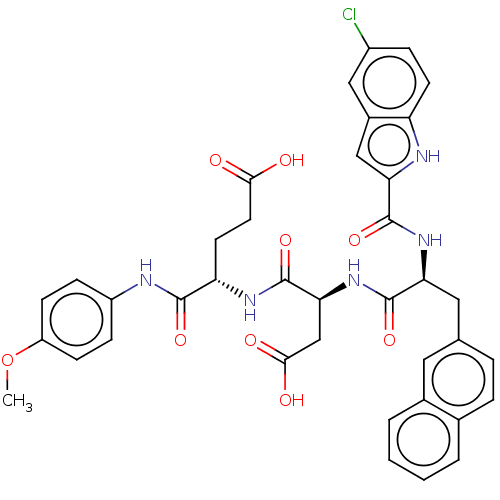 (CHEMBL4565782) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396288 (US10314832, Compound 11 | US10314832, Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM396296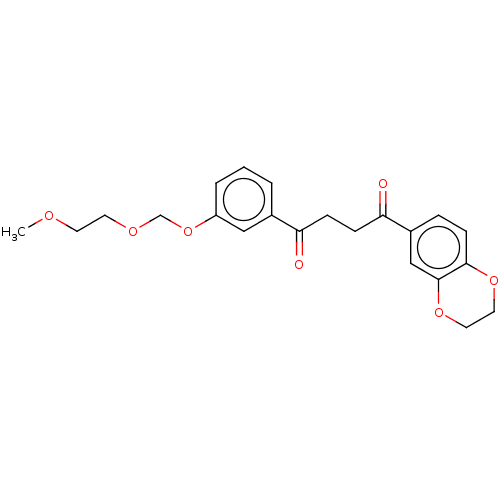 (US10314832, Compound 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Compounds that enhance the Wnt activity, or Activators, were assayed as follows. Reporter cell lines were generated by stably transducing cells of ca... | J Med Chem 52: 3523-38 (2009) BindingDB Entry DOI: 10.7270/Q2M047TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509799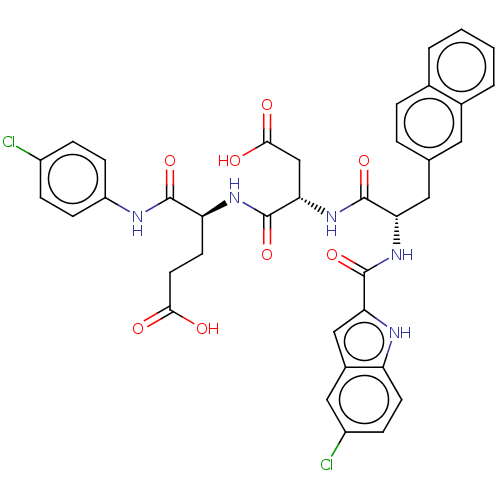 (CHEMBL4532791) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509807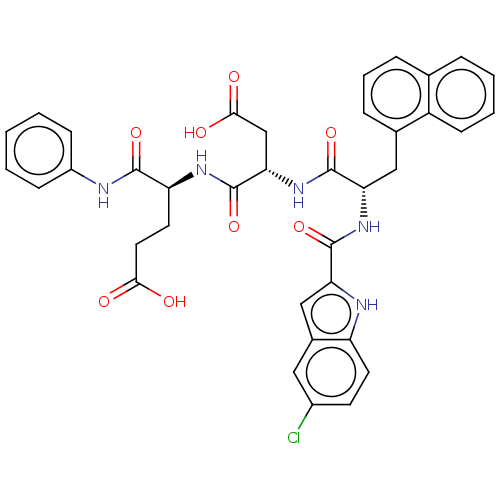 (CHEMBL4560197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50577925 (CHEMBL4846565) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of beta-catenin (unknown origin) transfected in HEK293 cells cotransfected with pCMV-RL assessed as reduction in luciferase activity measu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00742 BindingDB Entry DOI: 10.7270/Q2SN0DSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509829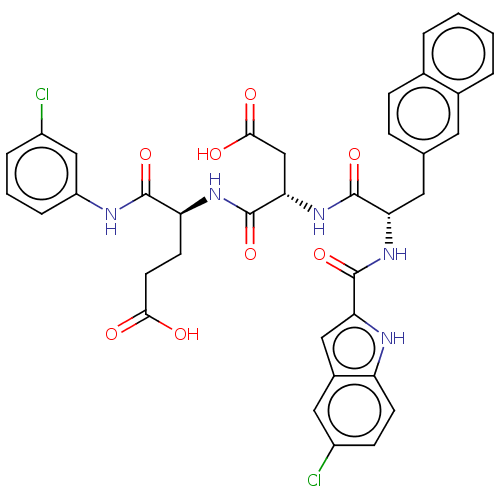 (CHEMBL4456139) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509833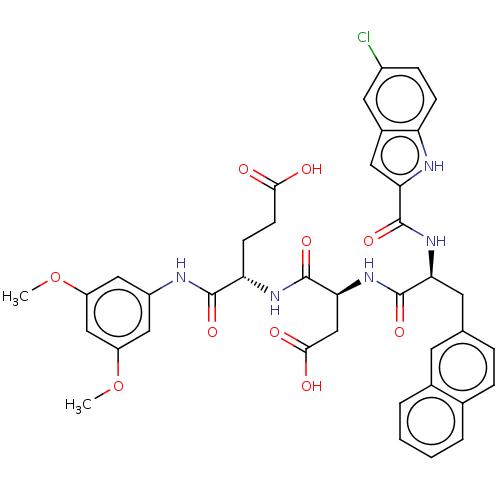 (CHEMBL4464915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509796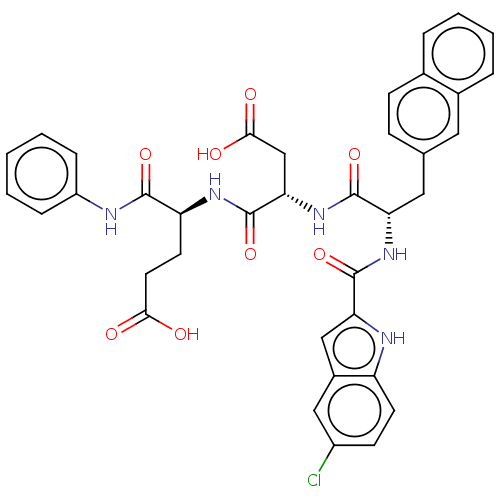 (CHEMBL4535419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509780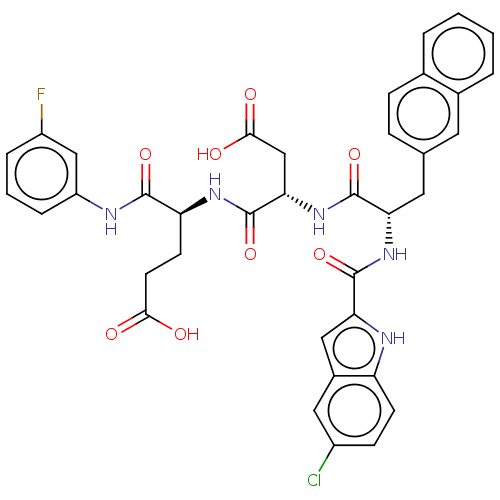 (CHEMBL4538548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50014132 (CHEMBL2323032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of human wild-type beta-catenin (residues 138-686)/C-terminally fluorescein labeled human wild-type Tcf4 (residues 7-51) interaction after... | J Med Chem 58: 4678-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00223 BindingDB Entry DOI: 10.7270/Q2G44S28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509798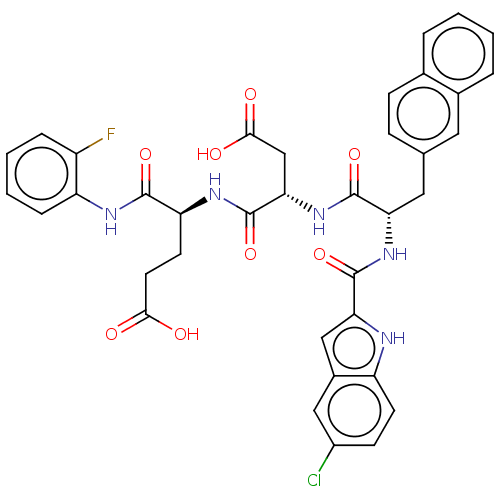 (CHEMBL4515538) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509805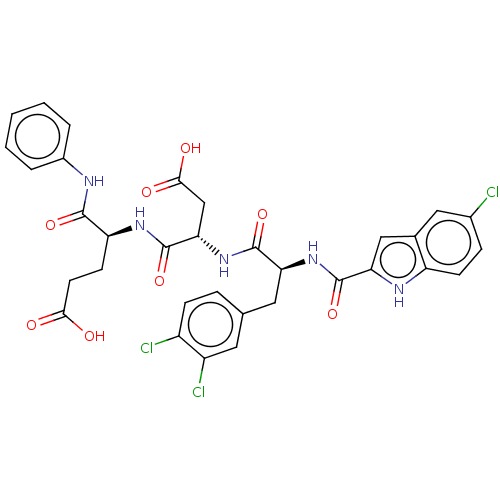 (CHEMBL4451874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50094490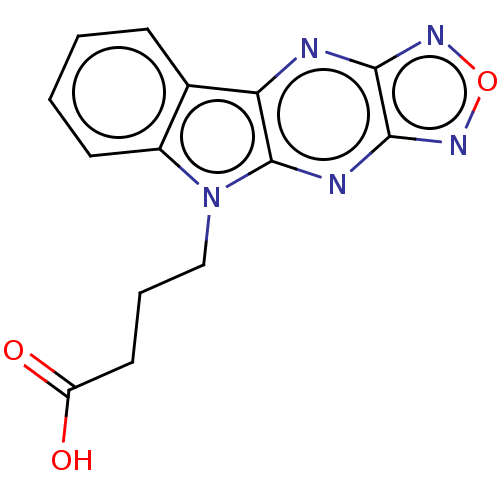 (CHEMBL3589156) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of human wild-type beta-catenin (residues 138-686)/C-terminally fluorescein labeled human wild-type Tcf4 (residues 7-51) interaction after... | J Med Chem 58: 4678-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00223 BindingDB Entry DOI: 10.7270/Q2G44S28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50014148 (CHEMBL3260853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50577931 (CHEMBL4863751) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of beta-catenin (unknown origin) transfected in HEK293 cells cotransfected with pCMV-RL assessed as reduction in luciferase activity measu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00742 BindingDB Entry DOI: 10.7270/Q2SN0DSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509823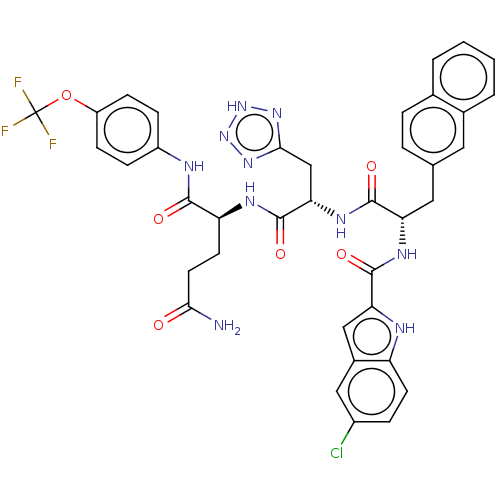 (CHEMBL4561695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50509827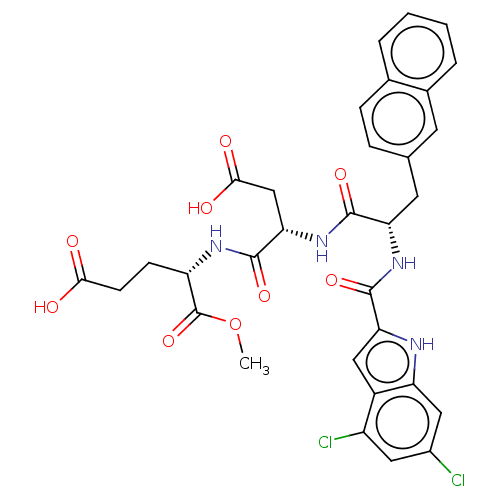 (CHEMBL4544508) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of C-terminal fluorescein-labeled human TCF4 (7 to 51 residues) binding to wild-type C-terminal His6-tagged beta-catenin (138 to 781 resid... | J Med Chem 62: 3617-3635 (2019) Article DOI: 10.1021/acs.jmedchem.9b00147 BindingDB Entry DOI: 10.7270/Q2KP85G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 119 total ) | Next | Last >> |