 Found 327 hits Enz. Inhib. hit(s) with all data for entry = 50004965
Found 327 hits Enz. Inhib. hit(s) with all data for entry = 50004965 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50536169
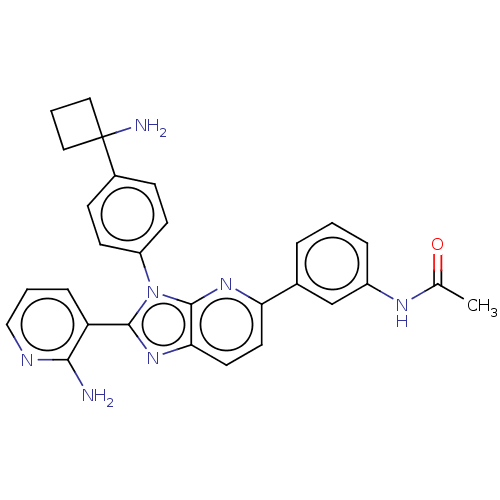
(CHEMBL4519558)Show SMILES Cl.CC(=O)Nc1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(cc3)C3(N)CCC3)c2n1 Show InChI InChI=1S/C29H27N7O/c1-18(37)33-21-6-2-5-19(17-21)24-12-13-25-28(34-24)36(27(35-25)23-7-3-16-32-26(23)30)22-10-8-20(9-11-22)29(31)14-4-15-29/h2-3,5-13,16-17H,4,14-15,31H2,1H3,(H2,30,32)(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50536172
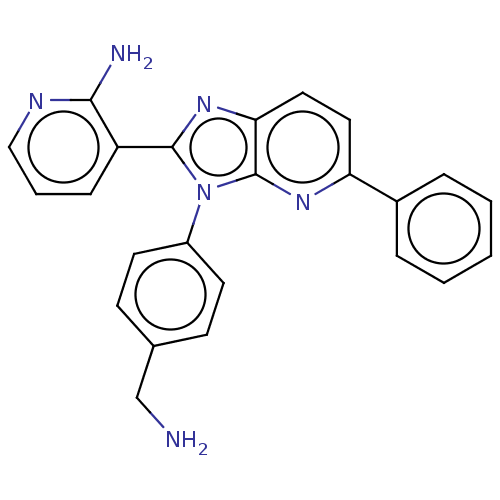
(CHEMBL4513257)Show SMILES Cl.NCc1ccc(cc1)-n1c(nc2ccc(nc12)-c1ccccc1)-c1cccnc1N Show InChI InChI=1S/C24H20N6/c25-15-16-8-10-18(11-9-16)30-23(19-7-4-14-27-22(19)26)29-21-13-12-20(28-24(21)30)17-5-2-1-3-6-17/h1-14H,15,25H2,(H2,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536172

(CHEMBL4513257)Show SMILES Cl.NCc1ccc(cc1)-n1c(nc2ccc(nc12)-c1ccccc1)-c1cccnc1N Show InChI InChI=1S/C24H20N6/c25-15-16-8-10-18(11-9-16)30-23(19-7-4-14-27-22(19)26)29-21-13-12-20(28-24(21)30)17-5-2-1-3-6-17/h1-14H,15,25H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50536173
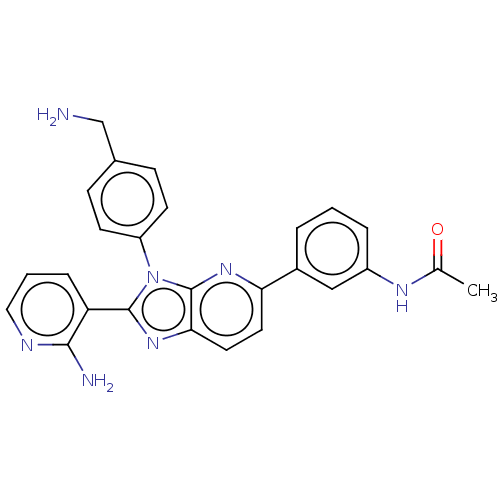
(CHEMBL4546484)Show SMILES Cl.CC(=O)Nc1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(CN)cc3)c2n1 Show InChI InChI=1S/C26H23N7O/c1-16(34)30-19-5-2-4-18(14-19)22-11-12-23-26(31-22)33(20-9-7-17(15-27)8-10-20)25(32-23)21-6-3-13-29-24(21)28/h2-14H,15,27H2,1H3,(H2,28,29)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397799

(CHEMBL2177825)Show SMILES Nc1ncccc1-c1nc2cc(Br)cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H19BrN6O/c26-18-13-21-24(29-15-18)32(23(31-21)20-7-4-12-28-22(20)27)19-10-8-16(9-11-19)14-30-25(33)17-5-2-1-3-6-17/h1-13,15H,14H2,(H2,27,28)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 in human AN3CA cells assessed as phosphorylation PRAS40 at T246 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397790
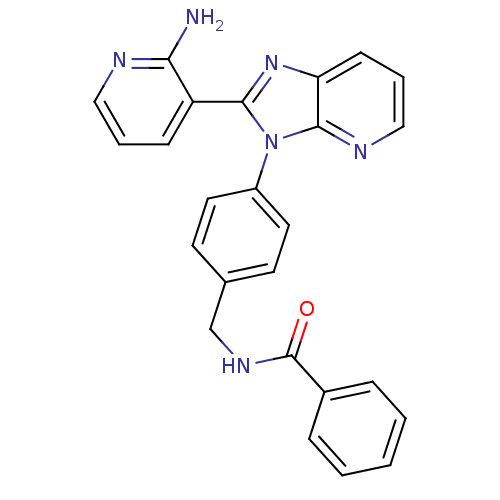
(CHEMBL2177819)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-22-20(8-4-14-27-22)23-30-21-9-5-15-28-24(21)31(23)19-12-10-17(11-13-19)16-29-25(32)18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 in human AN3CA cells assessed as phosphorylation PRAS40 at T246 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397788
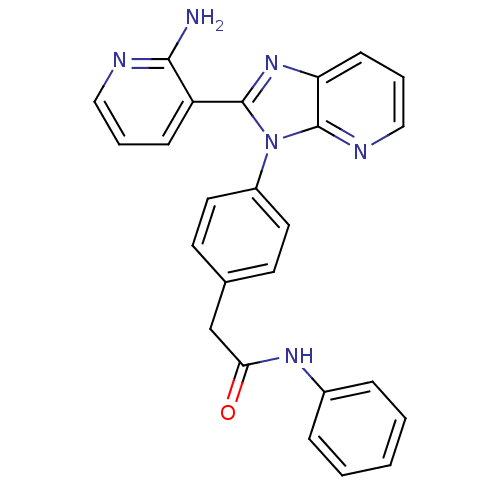
(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 in human AN3CA cells assessed as phosphorylation PRAS40 at T246 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397790

(CHEMBL2177819)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-22-20(8-4-14-27-22)23-30-21-9-5-15-28-24(21)31(23)19-12-10-17(11-13-19)16-29-25(32)18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 in human A2780 cells assessed as phosphorylation PRAS40 at T246 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397788

(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 in human A2780 cells assessed as phosphorylation PRAS40 at T246 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397793

(CHEMBL2177835)Show SMILES Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C31H24N6O/c32-28-25(12-7-19-33-28)29-36-27-18-17-26(22-8-3-1-4-9-22)35-30(27)37(29)24-15-13-21(14-16-24)20-34-31(38)23-10-5-2-6-11-23/h1-19H,20H2,(H2,32,33)(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt 1 in human AN3CA cells assessed as phosphorylation of Akt at T308 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397799

(CHEMBL2177825)Show SMILES Nc1ncccc1-c1nc2cc(Br)cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H19BrN6O/c26-18-13-21-24(29-15-18)32(23(31-21)20-7-4-12-28-22(20)27)19-10-8-16(9-11-19)14-30-25(33)17-5-2-1-3-6-17/h1-13,15H,14H2,(H2,27,28)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt 1 in human AN3CA cells assessed as phosphorylation of Akt at T308 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397790

(CHEMBL2177819)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-22-20(8-4-14-27-22)23-30-21-9-5-15-28-24(21)31(23)19-12-10-17(11-13-19)16-29-25(32)18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt 1 in human AN3CA cells assessed as phosphorylation of Akt at T308 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397788

(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt 1 in human AN3CA cells assessed as phosphorylation of Akt at T308 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397793

(CHEMBL2177835)Show SMILES Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C31H24N6O/c32-28-25(12-7-19-33-28)29-36-27-18-17-26(22-8-3-1-4-9-22)35-30(27)37(29)24-15-13-21(14-16-24)20-34-31(38)23-10-5-2-6-11-23/h1-19H,20H2,(H2,32,33)(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 in human AN3CA cells assessed as phosphorylation of Akt at S473 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397799

(CHEMBL2177825)Show SMILES Nc1ncccc1-c1nc2cc(Br)cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H19BrN6O/c26-18-13-21-24(29-15-18)32(23(31-21)20-7-4-12-28-22(20)27)19-10-8-16(9-11-19)14-30-25(33)17-5-2-1-3-6-17/h1-13,15H,14H2,(H2,27,28)(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt3 (1 to 479) expressed in Sf9 cells using biotin-GRPRTSSFAEG as substrate after 20 mins by Alpha-screen assay |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50397798
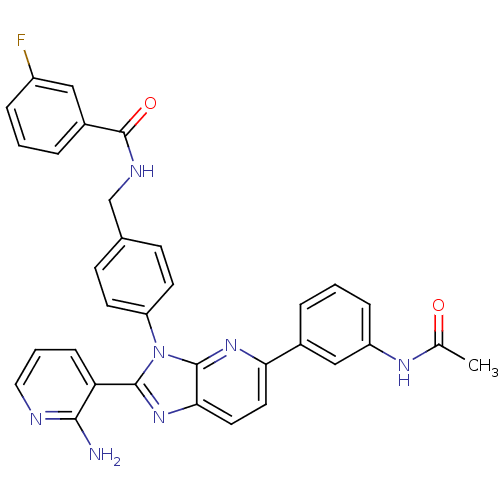
(CHEMBL2177836)Show SMILES CC(=O)Nc1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(CNC(=O)c4cccc(F)c4)cc3)c2n1 Show InChI InChI=1S/C33H26FN7O2/c1-20(42)38-25-8-3-5-22(18-25)28-14-15-29-32(39-28)41(31(40-29)27-9-4-16-36-30(27)35)26-12-10-21(11-13-26)19-37-33(43)23-6-2-7-24(34)17-23/h2-18H,19H2,1H3,(H2,35,36)(H,37,43)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50397780

(CHEMBL2177831)Show SMILES Nc1ncccc1-c1nc2cc(cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)-c1cccnc1 Show InChI InChI=1S/C30H23N7O/c31-27-25(9-5-15-33-27)28-36-26-16-23(22-8-4-14-32-18-22)19-34-29(26)37(28)24-12-10-20(11-13-24)17-35-30(38)21-6-2-1-3-7-21/h1-16,18-19H,17H2,(H2,31,33)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50397790

(CHEMBL2177819)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-22-20(8-4-14-27-22)23-30-21-9-5-15-28-24(21)31(23)19-12-10-17(11-13-19)16-29-25(32)18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50397788

(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50397798

(CHEMBL2177836)Show SMILES CC(=O)Nc1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(CNC(=O)c4cccc(F)c4)cc3)c2n1 Show InChI InChI=1S/C33H26FN7O2/c1-20(42)38-25-8-3-5-22(18-25)28-14-15-29-32(39-28)41(31(40-29)27-9-4-16-36-30(27)35)26-12-10-21(11-13-26)19-37-33(43)23-6-2-7-24(34)17-23/h2-18H,19H2,1H3,(H2,35,36)(H,37,43)(H,38,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50397780

(CHEMBL2177831)Show SMILES Nc1ncccc1-c1nc2cc(cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)-c1cccnc1 Show InChI InChI=1S/C30H23N7O/c31-27-25(9-5-15-33-27)28-36-26-16-23(22-8-4-14-32-18-22)19-34-29(26)37(28)24-12-10-20(11-13-24)17-35-30(38)21-6-2-1-3-7-21/h1-16,18-19H,17H2,(H2,31,33)(H,35,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50397790

(CHEMBL2177819)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-22-20(8-4-14-27-22)23-30-21-9-5-15-28-24(21)31(23)19-12-10-17(11-13-19)16-29-25(32)18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50397788

(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50397780

(CHEMBL2177831)Show SMILES Nc1ncccc1-c1nc2cc(cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)-c1cccnc1 Show InChI InChI=1S/C30H23N7O/c31-27-25(9-5-15-33-27)28-36-26-16-23(22-8-4-14-32-18-22)19-34-29(26)37(28)24-12-10-20(11-13-24)17-35-30(38)21-6-2-1-3-7-21/h1-16,18-19H,17H2,(H2,31,33)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50397790

(CHEMBL2177819)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-22-20(8-4-14-27-22)23-30-21-9-5-15-28-24(21)31(23)19-12-10-17(11-13-19)16-29-25(32)18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50397788

(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50397780

(CHEMBL2177831)Show SMILES Nc1ncccc1-c1nc2cc(cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)-c1cccnc1 Show InChI InChI=1S/C30H23N7O/c31-27-25(9-5-15-33-27)28-36-26-16-23(22-8-4-14-32-18-22)19-34-29(26)37(28)24-12-10-20(11-13-24)17-35-30(38)21-6-2-1-3-7-21/h1-16,18-19H,17H2,(H2,31,33)(H,35,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50397790

(CHEMBL2177819)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-22-20(8-4-14-27-22)23-30-21-9-5-15-28-24(21)31(23)19-12-10-17(11-13-19)16-29-25(32)18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50397788

(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50397780

(CHEMBL2177831)Show SMILES Nc1ncccc1-c1nc2cc(cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)-c1cccnc1 Show InChI InChI=1S/C30H23N7O/c31-27-25(9-5-15-33-27)28-36-26-16-23(22-8-4-14-32-18-22)19-34-29(26)37(28)24-12-10-20(11-13-24)17-35-30(38)21-6-2-1-3-7-21/h1-16,18-19H,17H2,(H2,31,33)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50397790

(CHEMBL2177819)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-22-20(8-4-14-27-22)23-30-21-9-5-15-28-24(21)31(23)19-12-10-17(11-13-19)16-29-25(32)18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50397788

(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50397798

(CHEMBL2177836)Show SMILES CC(=O)Nc1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(CNC(=O)c4cccc(F)c4)cc3)c2n1 Show InChI InChI=1S/C33H26FN7O2/c1-20(42)38-25-8-3-5-22(18-25)28-14-15-29-32(39-28)41(31(40-29)27-9-4-16-36-30(27)35)26-12-10-21(11-13-26)19-37-33(43)23-6-2-7-24(34)17-23/h2-18H,19H2,1H3,(H2,35,36)(H,37,43)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50397780

(CHEMBL2177831)Show SMILES Nc1ncccc1-c1nc2cc(cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)-c1cccnc1 Show InChI InChI=1S/C30H23N7O/c31-27-25(9-5-15-33-27)28-36-26-16-23(22-8-4-14-32-18-22)19-34-29(26)37(28)24-12-10-20(11-13-24)17-35-30(38)21-6-2-1-3-7-21/h1-16,18-19H,17H2,(H2,31,33)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50397790

(CHEMBL2177819)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-22-20(8-4-14-27-22)23-30-21-9-5-15-28-24(21)31(23)19-12-10-17(11-13-19)16-29-25(32)18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50397788

(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 mins |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397793

(CHEMBL2177835)Show SMILES Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C31H24N6O/c32-28-25(12-7-19-33-28)29-36-27-18-17-26(22-8-3-1-4-9-22)35-30(27)37(29)24-15-13-21(14-16-24)20-34-31(38)23-10-5-2-6-11-23/h1-19H,20H2,(H2,32,33)(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 in human AN3CA cells assessed as phosphorylation PRAS40 at T246 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397794

(CHEMBL2177827)Show SMILES CC(=O)Nc1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(CC(=O)Nc4ccccc4)cc3)c2n1 Show InChI InChI=1S/C33H27N7O2/c1-21(41)36-25-10-5-7-23(20-25)28-16-17-29-33(38-28)40(32(39-29)27-11-6-18-35-31(27)34)26-14-12-22(13-15-26)19-30(42)37-24-8-3-2-4-9-24/h2-18,20H,19H2,1H3,(H2,34,35)(H,36,41)(H,37,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 in human AN3CA cells assessed as phosphorylation PRAS40 at T246 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397785

(CHEMBL2177826)Show SMILES Nc1ncccc1-c1nc2cc(cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)C1CCCC1 Show InChI InChI=1S/C30H28N6O/c31-27-25(11-6-16-32-27)28-35-26-17-23(21-7-4-5-8-21)19-33-29(26)36(28)24-14-12-20(13-15-24)18-34-30(37)22-9-2-1-3-10-22/h1-3,6,9-17,19,21H,4-5,7-8,18H2,(H2,31,32)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 in human AN3CA cells assessed as phosphorylation PRAS40 at T246 after 2 hrs by Western Blot analysis |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50536170
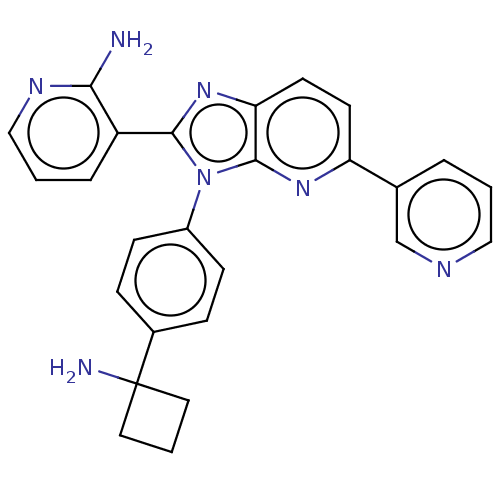
(CHEMBL4544109)Show SMILES Cl.Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(cc1)C1(N)CCC1)-c1cccnc1 Show InChI InChI=1S/C26H23N7/c27-23-20(5-2-15-30-23)24-32-22-11-10-21(17-4-1-14-29-16-17)31-25(22)33(24)19-8-6-18(7-9-19)26(28)12-3-13-26/h1-2,4-11,14-16H,3,12-13,28H2,(H2,27,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397785

(CHEMBL2177826)Show SMILES Nc1ncccc1-c1nc2cc(cnc2n1-c1ccc(CNC(=O)c2ccccc2)cc1)C1CCCC1 Show InChI InChI=1S/C30H28N6O/c31-27-25(11-6-16-32-27)28-35-26-17-23(21-7-4-5-8-21)19-33-29(26)36(28)24-14-12-20(13-15-24)18-34-30(37)22-9-2-1-3-10-22/h1-3,6,9-17,19,21H,4-5,7-8,18H2,(H2,31,32)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (1 to 480) expressed in Sf9 cells by indirect affinity mass spectrometry assay |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397808

(CHEMBL2177823)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1cccc(CNC(=O)c2ccccc2)c1 Show InChI InChI=1S/C25H20N6O/c26-22-20(11-5-13-27-22)23-30-21-12-6-14-28-24(21)31(23)19-10-4-7-17(15-19)16-29-25(32)18-8-2-1-3-9-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (1 to 480) expressed in Sf9 cells using biotin-GRPRTSSFAEG as substrate after 20 mins by Alpha-screen assay |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397788

(CHEMBL2177818)Show SMILES Nc1ncccc1-c1nc2cccnc2n1-c1ccc(CC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C25H20N6O/c26-23-20(8-4-14-27-23)24-30-21-9-5-15-28-25(21)31(24)19-12-10-17(11-13-19)16-22(32)29-18-6-2-1-3-7-18/h1-15H,16H2,(H2,26,27)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (1 to 480) expressed in Sf9 cells by indirect affinity mass spectrometry assay |
J Med Chem 55: 5291-310 (2012)
Article DOI: 10.1021/jm300276x
BindingDB Entry DOI: 10.7270/Q2NK3G5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50536167
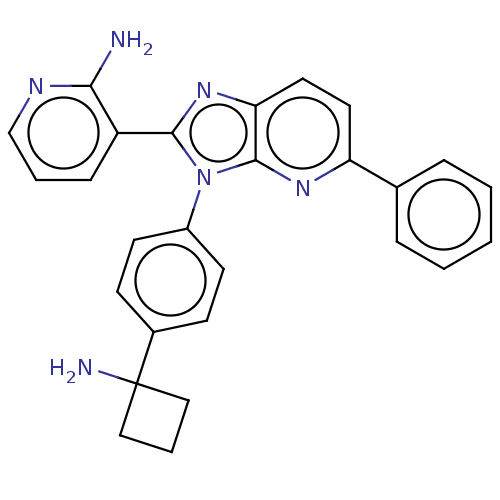
(CHEMBL4523032)Show SMILES Cl.Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(cc1)C1(N)CCC1)-c1ccccc1 Show InChI InChI=1S/C27H24N6/c28-24-21(8-4-17-30-24)25-32-23-14-13-22(18-6-2-1-3-7-18)31-26(23)33(25)20-11-9-19(10-12-20)27(29)15-5-16-27/h1-4,6-14,17H,5,15-16,29H2,(H2,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50397811
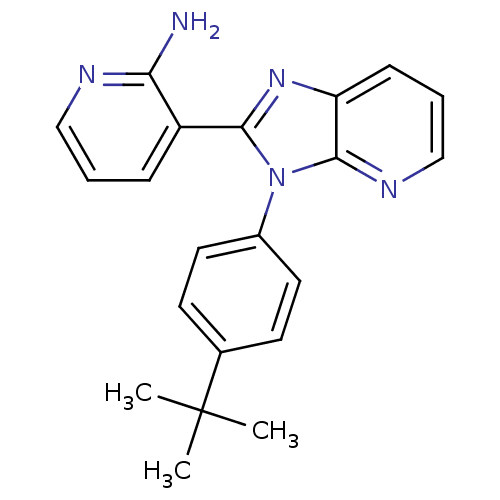
(CHEMBL2177840)Show SMILES CC(C)(C)c1ccc(cc1)-n1c(nc2cccnc12)-c1cccnc1N Show InChI InChI=1S/C21H21N5/c1-21(2,3)14-8-10-15(11-9-14)26-19(16-6-4-12-23-18(16)22)25-17-7-5-13-24-20(17)26/h4-13H,1-3H3,(H2,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length unphosphorylated AKT2 (1 to 481 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536170

(CHEMBL4544109)Show SMILES Cl.Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(cc1)C1(N)CCC1)-c1cccnc1 Show InChI InChI=1S/C26H23N7/c27-23-20(5-2-15-30-23)24-32-22-11-10-21(17-4-1-14-29-16-17)31-25(22)33(24)19-8-6-18(7-9-19)26(28)12-3-13-26/h1-2,4-11,14-16H,3,12-13,28H2,(H2,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536168
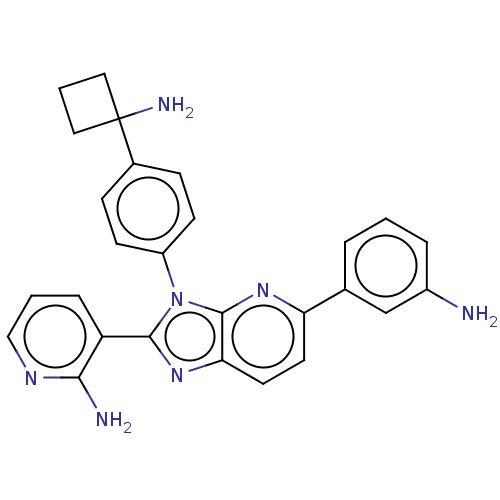
(CHEMBL4574111)Show SMILES Cl.Nc1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(cc3)C3(N)CCC3)c2n1 Show InChI InChI=1S/C27H25N7/c28-19-5-1-4-17(16-19)22-11-12-23-26(32-22)34(25(33-23)21-6-2-15-31-24(21)29)20-9-7-18(8-10-20)27(30)13-3-14-27/h1-2,4-12,15-16H,3,13-14,28,30H2,(H2,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50536180
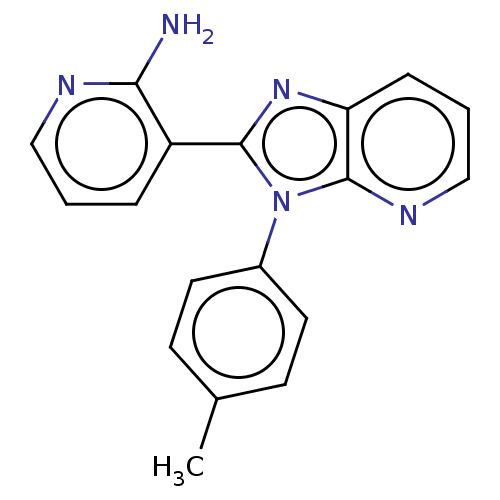
(CHEMBL4535365)Show InChI InChI=1S/C18H15N5/c1-12-6-8-13(9-7-12)23-17(14-4-2-10-20-16(14)19)22-15-5-3-11-21-18(15)23/h2-11H,1H3,(H2,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length unphosphorylated AKT2 (1 to 481 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50536167

(CHEMBL4523032)Show SMILES Cl.Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(cc1)C1(N)CCC1)-c1ccccc1 Show InChI InChI=1S/C27H24N6/c28-24-21(8-4-17-30-24)25-32-23-14-13-22(18-6-2-1-3-7-18)31-26(23)33(25)20-11-9-19(10-12-20)27(29)15-5-16-27/h1-4,6-14,17H,5,15-16,29H2,(H2,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50536167

(CHEMBL4523032)Show SMILES Cl.Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(cc1)C1(N)CCC1)-c1ccccc1 Show InChI InChI=1S/C27H24N6/c28-24-21(8-4-17-30-24)25-32-23-14-13-22(18-6-2-1-3-7-18)31-26(23)33(25)20-11-9-19(10-12-20)27(29)15-5-16-27/h1-4,6-14,17H,5,15-16,29H2,(H2,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins by LC/MS/MS analysis |
J Med Chem 59: 6455-69 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00619
BindingDB Entry DOI: 10.7270/Q2RN3CCM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data