 Found 655 hits Enz. Inhib. hit(s) with all data for entry = 50004968
Found 655 hits Enz. Inhib. hit(s) with all data for entry = 50004968 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127923

(5-{4-[3-(4-Phenoxy-2-propyl-phenoxy)-propoxy]-3-pr...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc(cc1CCC)-c1sc(=O)[nH]c1O Show InChI InChI=1S/C30H33NO5S/c1-3-9-21-19-23(28-29(32)31-30(33)37-28)13-15-26(21)34-17-8-18-35-27-16-14-25(20-22(27)10-4-2)36-24-11-6-5-7-12-24/h5-7,11-16,19-20,32H,3-4,8-10,17-18H2,1-2H3,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPAR gamma (peroxisome proliferator-activated gamma receptor) |
Bioorg Med Chem Lett 13: 1801-4 (2003)
BindingDB Entry DOI: 10.7270/Q2H994K2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148100
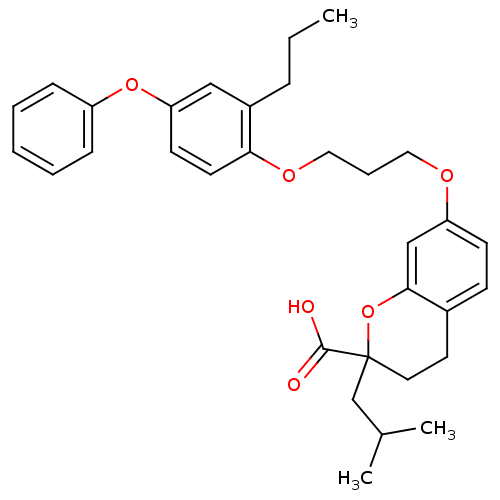
(2-tert-Butyl-7-[3-(4-phenoxy-2-propyl-phenoxy)-pro...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc2CCC(CC(C)C)(Oc2c1)C(O)=O Show InChI InChI=1S/C32H38O6/c1-4-9-25-20-28(37-26-10-6-5-7-11-26)14-15-29(25)36-19-8-18-35-27-13-12-24-16-17-32(31(33)34,22-23(2)3)38-30(24)21-27/h5-7,10-15,20-21,23H,4,8-9,16-19,22H2,1-3H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor gamma using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148106

(2-Ethyl-7-{3-[4-(4-fluoro-phenoxy)-2-propyl-phenox...)Show SMILES CCCc1cc(Oc2ccc(F)cc2)ccc1OCCCOc1ccc2CCC(CC)(Oc2c1)C(O)=O Show InChI InChI=1S/C30H33FO6/c1-3-6-22-19-26(36-24-11-8-23(31)9-12-24)13-14-27(22)35-18-5-17-34-25-10-7-21-15-16-30(4-2,29(32)33)37-28(21)20-25/h7-14,19-20H,3-6,15-18H2,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor gamma using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504051

(CHEMBL4450502)Show SMILES CC1=CC[C@]2(C[C@@H]1O)[C@@H](CC[C@@H]2C(O)=O)C1CCCCC1 |r,t:1| Show InChI InChI=1S/C18H28O3/c1-12-9-10-18(11-16(12)19)14(7-8-15(18)17(20)21)13-5-3-2-4-6-13/h9,13-16,19H,2-8,10-11H2,1H3,(H,20,21)/t14-,15+,16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168552

((R)-7-[3-(2-Chloro-4-ethoxy-phenoxy)-propoxy]-2-me...)Show SMILES CCOc1ccc(OCCCOc2ccc3CC[C@@](C)(Oc3c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C22H25ClO6/c1-3-26-16-7-8-19(18(23)13-16)28-12-4-11-27-17-6-5-15-9-10-22(2,21(24)25)29-20(15)14-17/h5-8,13-14H,3-4,9-12H2,1-2H3,(H,24,25)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148092
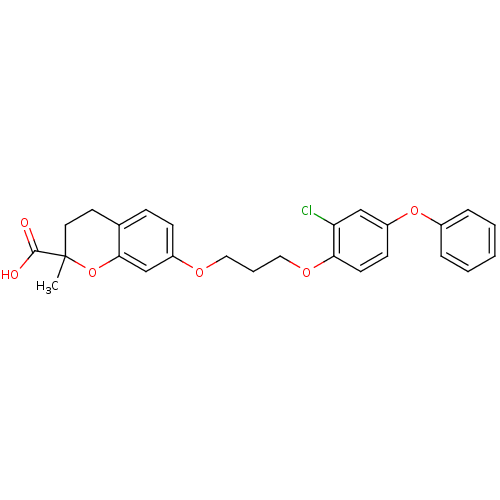
(7-[3-(2-Chloro-4-phenoxy-phenoxy)-propoxy]-2-methy...)Show SMILES CC1(CCc2ccc(OCCCOc3ccc(Oc4ccccc4)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C26H25ClO6/c1-26(25(28)29)13-12-18-8-9-20(17-24(18)33-26)30-14-5-15-31-23-11-10-21(16-22(23)27)32-19-6-3-2-4-7-19/h2-4,6-11,16-17H,5,12-15H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor gamma using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148102
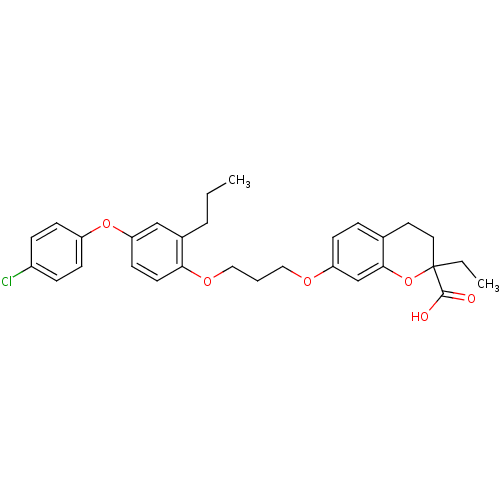
(7-{3-[4-(4-Chloro-phenoxy)-2-propyl-phenoxy]-propo...)Show SMILES CCCc1cc(Oc2ccc(Cl)cc2)ccc1OCCCOc1ccc2CCC(CC)(Oc2c1)C(O)=O Show InChI InChI=1S/C30H33ClO6/c1-3-6-22-19-26(36-24-11-8-23(31)9-12-24)13-14-27(22)35-18-5-17-34-25-10-7-21-15-16-30(4-2,29(32)33)37-28(21)20-25/h7-14,19-20H,3-6,15-18H2,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor gamma using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168544
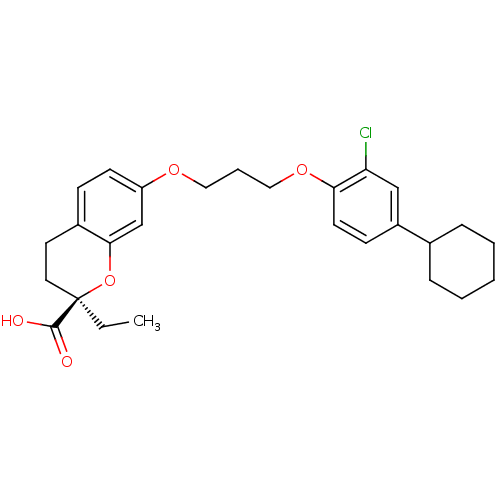
((R)-7-[3-(2-Chloro-4-cyclohexyl-phenoxy)-propoxy]-...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C3CCCCC3)cc2O1)C(O)=O Show InChI InChI=1S/C27H33ClO5/c1-2-27(26(29)30)14-13-20-9-11-22(18-25(20)33-27)31-15-6-16-32-24-12-10-21(17-23(24)28)19-7-4-3-5-8-19/h9-12,17-19H,2-8,13-16H2,1H3,(H,29,30)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148098
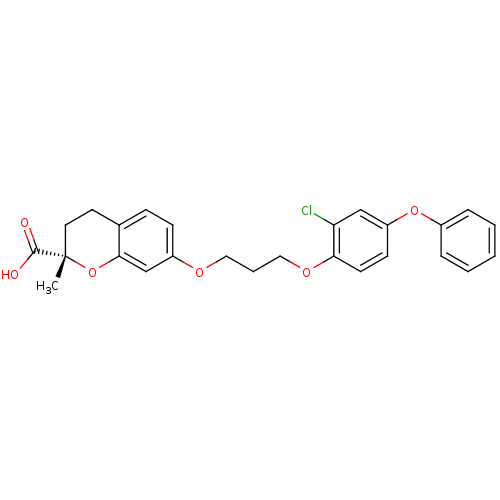
((S)-7-[3-(2-Chloro-4-phenoxy-phenoxy)-propoxy]-2-m...)Show SMILES C[C@]1(CCc2ccc(OCCCOc3ccc(Oc4ccccc4)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C26H25ClO6/c1-26(25(28)29)13-12-18-8-9-20(17-24(18)33-26)30-14-5-15-31-23-11-10-21(16-22(23)27)32-19-6-3-2-4-7-19/h2-4,6-11,16-17H,5,12-15H2,1H3,(H,28,29)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human PPAR gamma receptor using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148108
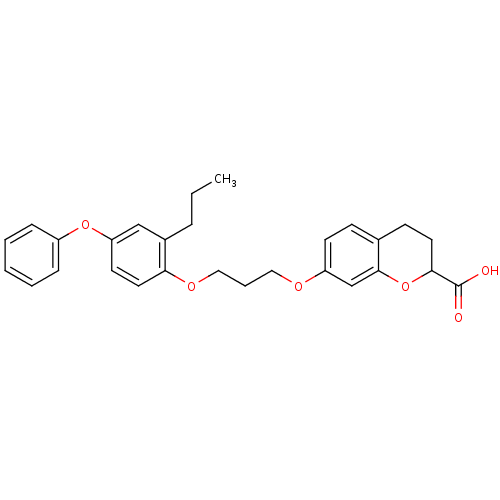
(7-[3-(4-Phenoxy-2-propyl-phenoxy)-propoxy]-chroman...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc2CCC(Oc2c1)C(O)=O Show InChI InChI=1S/C28H30O6/c1-2-7-21-18-24(33-22-8-4-3-5-9-22)13-15-25(21)32-17-6-16-31-23-12-10-20-11-14-26(28(29)30)34-27(20)19-23/h3-5,8-10,12-13,15,18-19,26H,2,6-7,11,14,16-17H2,1H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168568
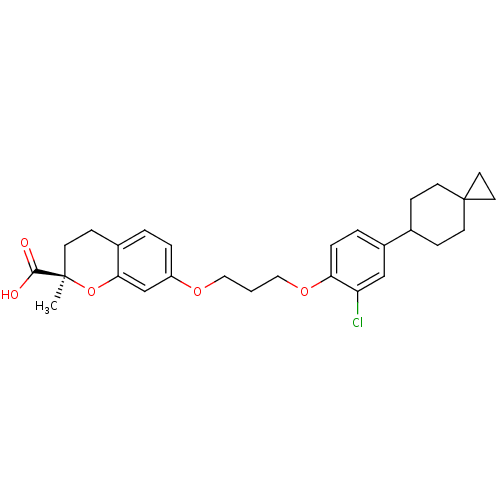
((R)-7-[3-(2-Chloro-4-spiro[2.5]oct-6-yl-phenoxy)-p...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C3CCC4(CC4)CC3)cc2O1)C(O)=O Show InChI InChI=1S/C28H33ClO5/c1-27(26(30)31)10-7-20-3-5-22(18-25(20)34-27)32-15-2-16-33-24-6-4-21(17-23(24)29)19-8-11-28(12-9-19)13-14-28/h3-6,17-19H,2,7-16H2,1H3,(H,30,31)/t27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168550
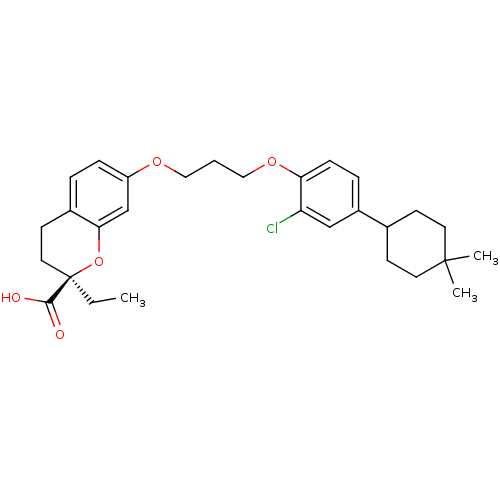
((R)-7-{3-[2-Chloro-4-(4,4-dimethyl-cyclohexyl)-phe...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C3CCC(C)(C)CC3)cc2O1)C(O)=O Show InChI InChI=1S/C29H37ClO5/c1-4-29(27(31)32)15-12-21-6-8-23(19-26(21)35-29)33-16-5-17-34-25-9-7-22(18-24(25)30)20-10-13-28(2,3)14-11-20/h6-9,18-20H,4-5,10-17H2,1-3H3,(H,31,32)/t29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148101
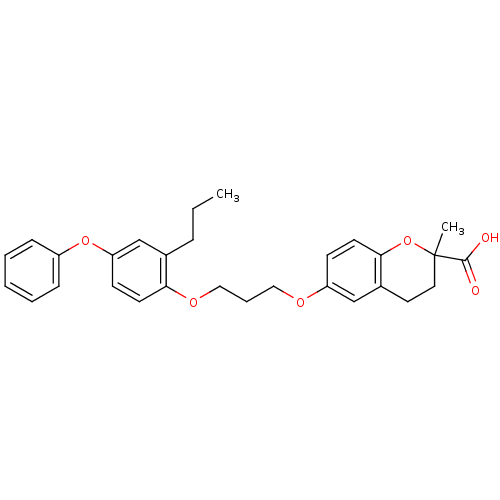
(2-Methyl-6-[3-(4-phenoxy-2-propyl-phenoxy)-propoxy...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc2OC(C)(CCc2c1)C(O)=O Show InChI InChI=1S/C29H32O6/c1-3-8-21-20-25(34-23-9-5-4-6-10-23)12-13-26(21)33-18-7-17-32-24-11-14-27-22(19-24)15-16-29(2,35-27)28(30)31/h4-6,9-14,19-20H,3,7-8,15-18H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor gamma using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148104
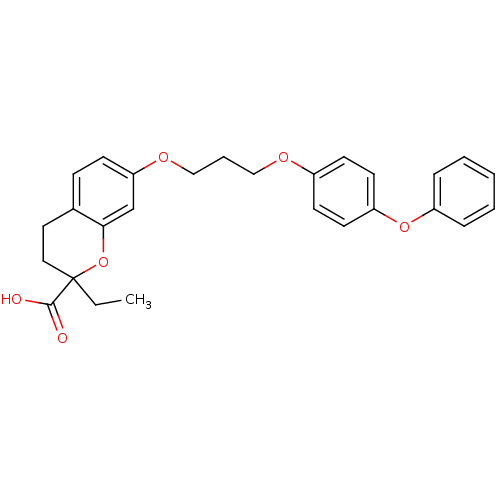
(2-Ethyl-7-[3-(4-phenoxy-phenoxy)-propoxy]-chroman-...)Show SMILES CCC1(CCc2ccc(OCCCOc3ccc(Oc4ccccc4)cc3)cc2O1)C(O)=O Show InChI InChI=1S/C27H28O6/c1-2-27(26(28)29)16-15-20-9-10-24(19-25(20)33-27)31-18-6-17-30-21-11-13-23(14-12-21)32-22-7-4-3-5-8-22/h3-5,7-14,19H,2,6,15-18H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168561
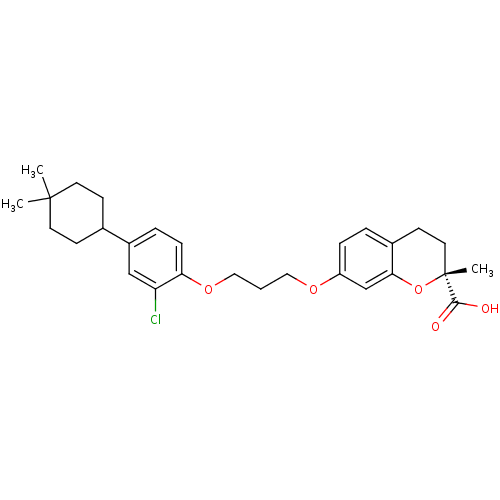
((R)-7-{3-[2-Chloro-4-(4,4-dimethyl-cyclohexyl)-phe...)Show SMILES CC1(C)CCC(CC1)c1ccc(OCCCOc2ccc3CC[C@@](C)(Oc3c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C28H35ClO5/c1-27(2)12-9-19(10-13-27)21-6-8-24(23(29)17-21)33-16-4-15-32-22-7-5-20-11-14-28(3,26(30)31)34-25(20)18-22/h5-8,17-19H,4,9-16H2,1-3H3,(H,30,31)/t28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168555
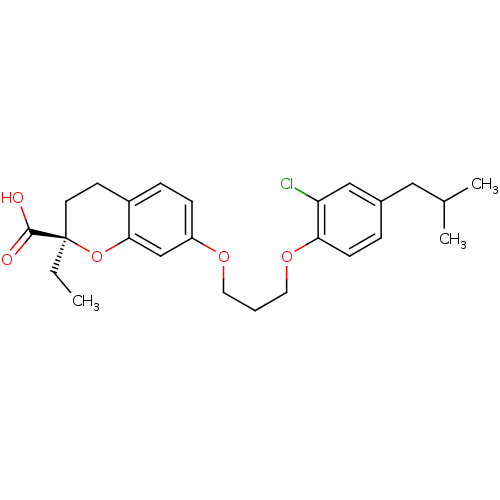
((R)-7-[3-(2-Chloro-4-isobutyl-phenoxy)-propoxy]-2-...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(CC(C)C)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C25H31ClO5/c1-4-25(24(27)28)11-10-19-7-8-20(16-23(19)31-25)29-12-5-13-30-22-9-6-18(14-17(2)3)15-21(22)26/h6-9,15-17H,4-5,10-14H2,1-3H3,(H,27,28)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50127929

(5-(4-{3-[2-Propyl-4-(4-trifluoromethyl-phenoxy)-ph...)Show SMILES CCCc1cc(Oc2ccc(cc2)C(F)(F)F)ccc1OCCCOc1ccc(cc1)-c1sc(=O)[nH]c1O Show InChI InChI=1S/C28H26F3NO5S/c1-2-4-19-17-23(37-22-11-7-20(8-12-22)28(29,30)31)13-14-24(19)36-16-3-15-35-21-9-5-18(6-10-21)25-26(33)32-27(34)38-25/h5-14,17,33H,2-4,15-16H2,1H3,(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPAR gamma (peroxisome proliferator-activated gamma receptor) |
Bioorg Med Chem Lett 13: 1801-4 (2003)
BindingDB Entry DOI: 10.7270/Q2H994K2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148099
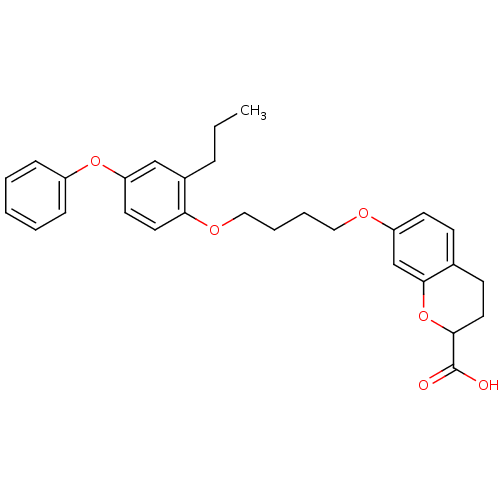
(7-[4-(4-Phenoxy-2-propyl-phenoxy)-butoxy]-chroman-...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCCOc1ccc2CCC(Oc2c1)C(O)=O Show InChI InChI=1S/C29H32O6/c1-2-8-22-19-25(34-23-9-4-3-5-10-23)14-16-26(22)33-18-7-6-17-32-24-13-11-21-12-15-27(29(30)31)35-28(21)20-24/h3-5,9-11,13-14,16,19-20,27H,2,6-8,12,15,17-18H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor gamma using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148089
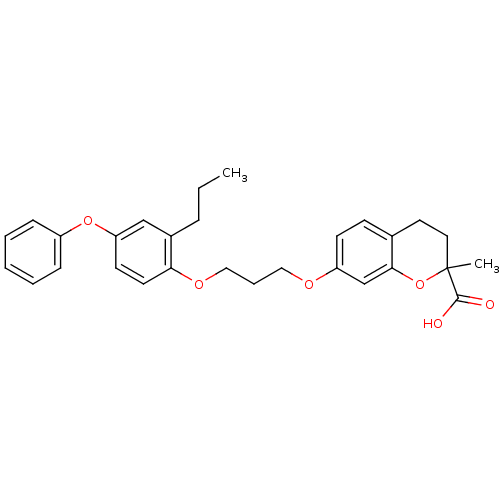
(2-Methyl-7-[3-(4-phenoxy-2-propyl-phenoxy)-propoxy...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc2CCC(C)(Oc2c1)C(O)=O Show InChI InChI=1S/C29H32O6/c1-3-8-22-19-25(34-23-9-5-4-6-10-23)13-14-26(22)33-18-7-17-32-24-12-11-21-15-16-29(2,28(30)31)35-27(21)20-24/h4-6,9-14,19-20H,3,7-8,15-18H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50168572

((R)-7-[3-(2-Chloro-4-trifluoromethoxy-phenoxy)-pro...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(OC(F)(F)F)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C22H22ClF3O6/c1-2-21(20(27)28)9-8-14-4-5-15(13-19(14)32-21)29-10-3-11-30-18-7-6-16(12-17(18)23)31-22(24,25)26/h4-7,12-13H,2-3,8-11H2,1H3,(H,27,28)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARdelta |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168563
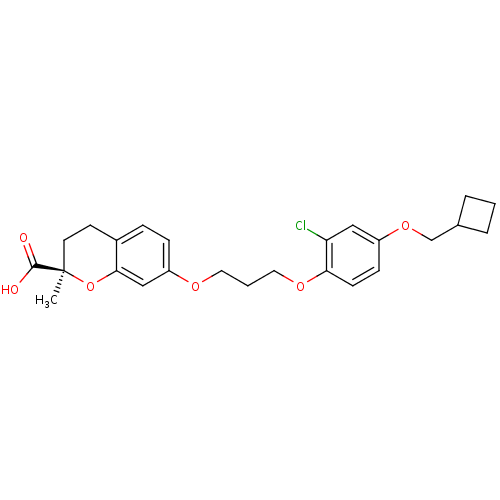
((R)-7-[3-(2-Chloro-4-cyclobutylmethoxy-phenoxy)-pr...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(OCC4CCC4)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C25H29ClO6/c1-25(24(27)28)11-10-18-6-7-20(15-23(18)32-25)29-12-3-13-30-22-9-8-19(14-21(22)26)31-16-17-4-2-5-17/h6-9,14-15,17H,2-5,10-13,16H2,1H3,(H,27,28)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50127919
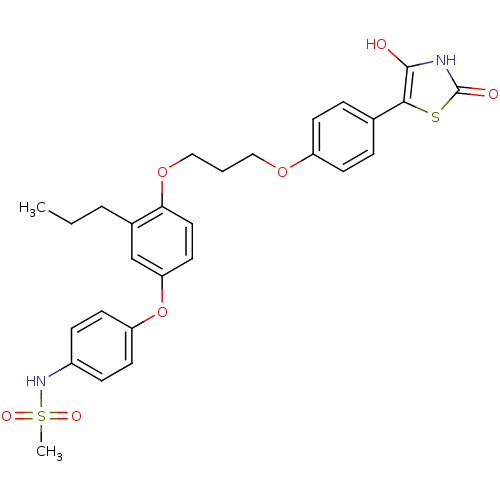
(CHEMBL53688 | N-[4-(4-{3-[4-(2,4-Dioxo-thiazolidin...)Show SMILES CCCc1cc(Oc2ccc(NS(C)(=O)=O)cc2)ccc1OCCCOc1ccc(cc1)-c1sc(=O)[nH]c1O Show InChI InChI=1S/C28H30N2O7S2/c1-3-5-20-18-24(37-23-12-8-21(9-13-23)30-39(2,33)34)14-15-25(20)36-17-4-16-35-22-10-6-19(7-11-22)26-27(31)29-28(32)38-26/h6-15,18,30-31H,3-5,16-17H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPAR gamma (peroxisome proliferator-activated gamma receptor) |
Bioorg Med Chem Lett 13: 1801-4 (2003)
BindingDB Entry DOI: 10.7270/Q2H994K2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50127920
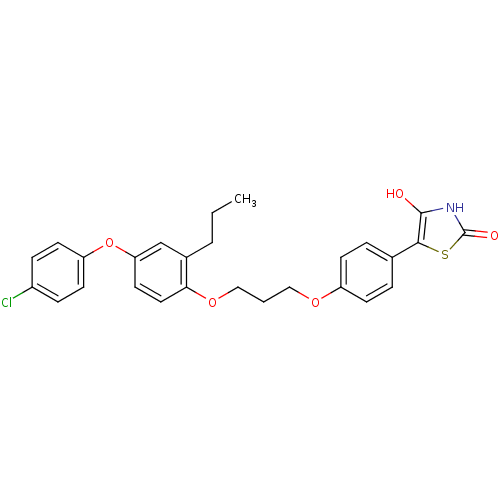
(5-(4-{3-[4-(4-Chloro-phenoxy)-2-propyl-phenoxy]-pr...)Show SMILES CCCc1cc(Oc2ccc(Cl)cc2)ccc1OCCCOc1ccc(cc1)-c1sc(=O)[nH]c1O Show InChI InChI=1S/C27H26ClNO5S/c1-2-4-19-17-23(34-22-11-7-20(28)8-12-22)13-14-24(19)33-16-3-15-32-21-9-5-18(6-10-21)25-26(30)29-27(31)35-25/h5-14,17,30H,2-4,15-16H2,1H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPAR gamma (peroxisome proliferator-activated gamma receptor) |
Bioorg Med Chem Lett 13: 1801-4 (2003)
BindingDB Entry DOI: 10.7270/Q2H994K2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50127917
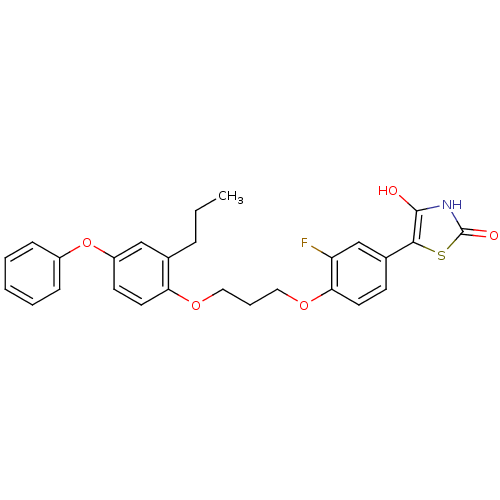
(5-{3-Fluoro-4-[3-(4-phenoxy-2-propyl-phenoxy)-prop...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc(cc1F)-c1sc(=O)[nH]c1O Show InChI InChI=1S/C27H26FNO5S/c1-2-7-18-16-21(34-20-8-4-3-5-9-20)11-13-23(18)32-14-6-15-33-24-12-10-19(17-22(24)28)25-26(30)29-27(31)35-25/h3-5,8-13,16-17,30H,2,6-7,14-15H2,1H3,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPAR delta (peroxisome proliferator-activated delta receptor) |
Bioorg Med Chem Lett 13: 1801-4 (2003)
BindingDB Entry DOI: 10.7270/Q2H994K2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148095
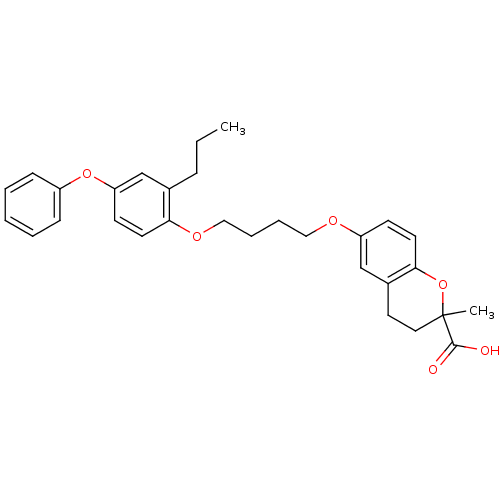
(2-Methyl-6-[4-(4-phenoxy-2-propyl-phenoxy)-butoxy]...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCCOc1ccc2OC(C)(CCc2c1)C(O)=O Show InChI InChI=1S/C30H34O6/c1-3-9-22-21-26(35-24-10-5-4-6-11-24)13-14-27(22)34-19-8-7-18-33-25-12-15-28-23(20-25)16-17-30(2,36-28)29(31)32/h4-6,10-15,20-21H,3,7-9,16-19H2,1-2H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29864
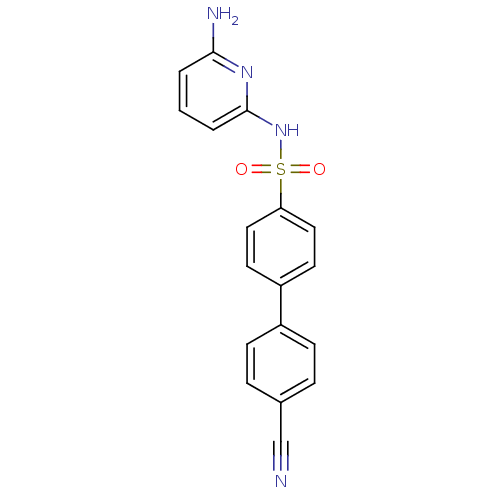
(N-(Pyridin-2-yl) arylsulfonamide, 26)Show SMILES Nc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C18H14N4O2S/c19-12-13-4-6-14(7-5-13)15-8-10-16(11-9-15)25(23,24)22-18-3-1-2-17(20)21-18/h1-11H,(H3,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148109
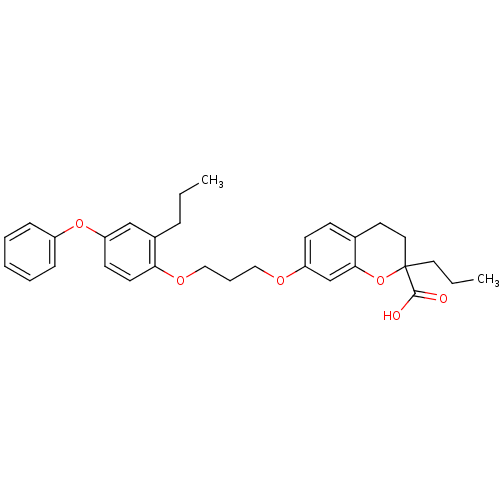
(7-[3-(4-Phenoxy-2-propyl-phenoxy)-propoxy]-2-propy...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc2CCC(CCC)(Oc2c1)C(O)=O Show InChI InChI=1S/C31H36O6/c1-3-9-24-21-27(36-25-10-6-5-7-11-25)14-15-28(24)35-20-8-19-34-26-13-12-23-16-18-31(17-4-2,30(32)33)37-29(23)22-26/h5-7,10-15,21-22H,3-4,8-9,16-20H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504050

(CHEMBL4436767)Show SMILES CC1=CC[C@]2(C=C1)[C@@H](CC[C@@H]2C(O)=O)C1CCCCC1 |r,c:5,t:1| Show InChI InChI=1S/C18H26O2/c1-13-9-11-18(12-10-13)15(7-8-16(18)17(19)20)14-5-3-2-4-6-14/h9-11,14-16H,2-8,12H2,1H3,(H,19,20)/t15-,16+,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148095

(2-Methyl-6-[4-(4-phenoxy-2-propyl-phenoxy)-butoxy]...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCCOc1ccc2OC(C)(CCc2c1)C(O)=O Show InChI InChI=1S/C30H34O6/c1-3-9-22-21-26(35-24-10-5-4-6-11-24)13-14-27(22)34-19-8-7-18-33-25-12-15-28-23(20-25)16-17-30(2,36-28)29(31)32/h4-6,10-15,20-21H,3,7-9,16-19H2,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor gamma using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50168565

((R)-7-[3-(2-Chloro-4-trifluoromethyl-phenoxy)-prop...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C(F)(F)F)cc2O1)C(O)=O Show InChI InChI=1S/C22H22ClF3O5/c1-2-21(20(27)28)9-8-14-4-6-16(13-19(14)31-21)29-10-3-11-30-18-7-5-15(12-17(18)23)22(24,25)26/h4-7,12-13H,2-3,8-11H2,1H3,(H,27,28)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARdelta |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50127927

(5-{4-[3-(2-Propyl-4-p-tolyloxy-phenoxy)-propoxy]-p...)Show SMILES CCCc1cc(Oc2ccc(C)cc2)ccc1OCCCOc1ccc(cc1)-c1sc(=O)[nH]c1O Show InChI InChI=1S/C28H29NO5S/c1-3-5-21-18-24(34-23-10-6-19(2)7-11-23)14-15-25(21)33-17-4-16-32-22-12-8-20(9-13-22)26-27(30)29-28(31)35-26/h6-15,18,30H,3-5,16-17H2,1-2H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPAR gamma (peroxisome proliferator-activated gamma receptor) |
Bioorg Med Chem Lett 13: 1801-4 (2003)
BindingDB Entry DOI: 10.7270/Q2H994K2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168572

((R)-7-[3-(2-Chloro-4-trifluoromethoxy-phenoxy)-pro...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(OC(F)(F)F)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C22H22ClF3O6/c1-2-21(20(27)28)9-8-14-4-5-15(13-19(14)32-21)29-10-3-11-30-18-7-6-16(12-17(18)23)31-22(24,25)26/h4-7,12-13H,2-3,8-11H2,1H3,(H,27,28)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50284638
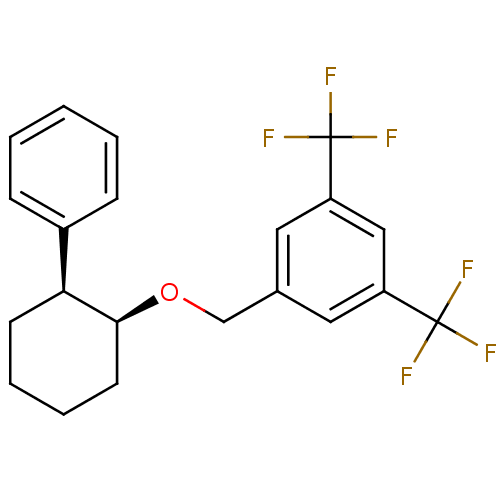
(1-((1S,2S)-2-Phenyl-cyclohexyloxymethyl)-3,5-bis-t...)Show SMILES FC(F)(F)c1cc(CO[C@H]2CCCC[C@H]2c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C21H20F6O/c22-20(23,24)16-10-14(11-17(12-16)21(25,26)27)13-28-19-9-5-4-8-18(19)15-6-2-1-3-7-15/h1-3,6-7,10-12,18-19H,4-5,8-9,13H2/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Neurokinin -1(NK-1) receptor of human by using [125I]- Tyr8 substance P as a radioligand in CHO cells |
Bioorg Med Chem Lett 5: 1345-1350 (1995)
Article DOI: 10.1016/0960-894X(95)00220-N
BindingDB Entry DOI: 10.7270/Q2416X13 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168569
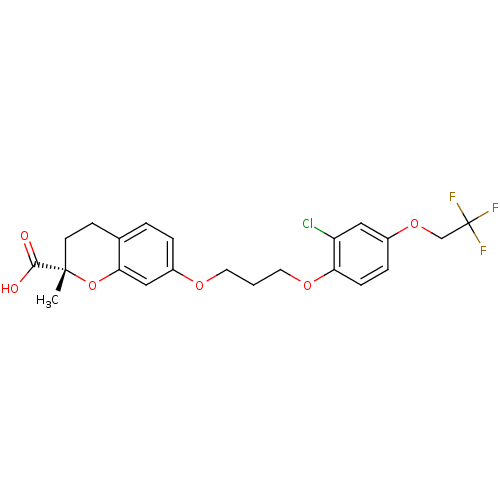
((S)-7-{3-[2-Chloro-4-(2,2,2-trifluoro-ethoxy)-phen...)Show SMILES C[C@]1(CCc2ccc(OCCCOc3ccc(OCC(F)(F)F)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C22H22ClF3O6/c1-21(20(27)28)8-7-14-3-4-16(12-19(14)32-21)29-9-2-10-30-18-6-5-15(11-17(18)23)31-13-22(24,25)26/h3-6,11-12H,2,7-10,13H2,1H3,(H,27,28)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168556
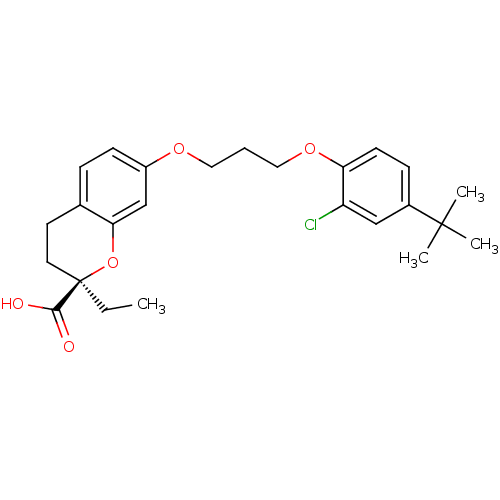
((R)-7-[3-(4-tert-Butyl-2-chloro-phenoxy)-propoxy]-...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C(C)(C)C)cc2O1)C(O)=O Show InChI InChI=1S/C25H31ClO5/c1-5-25(23(27)28)12-11-17-7-9-19(16-22(17)31-25)29-13-6-14-30-21-10-8-18(15-20(21)26)24(2,3)4/h7-10,15-16H,5-6,11-14H2,1-4H3,(H,27,28)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168559
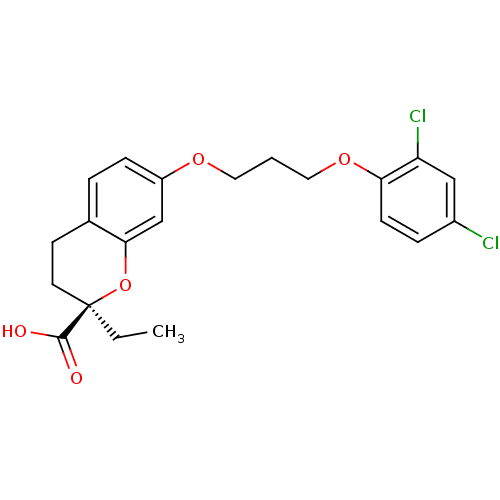
((R)-7-[3-(2,4-Dichloro-phenoxy)-propoxy]-2-ethyl-c...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(Cl)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C21H22Cl2O5/c1-2-21(20(24)25)9-8-14-4-6-16(13-19(14)28-21)26-10-3-11-27-18-7-5-15(22)12-17(18)23/h4-7,12-13H,2-3,8-11H2,1H3,(H,24,25)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148103
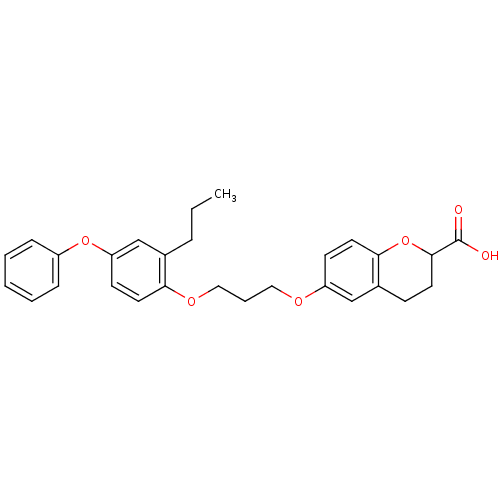
(6-[3-(4-Phenoxy-2-propyl-phenoxy)-propoxy]-chroman...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc2OC(CCc2c1)C(O)=O Show InChI InChI=1S/C28H30O6/c1-2-7-20-19-24(33-22-8-4-3-5-9-22)12-14-25(20)32-17-6-16-31-23-11-15-26-21(18-23)10-13-27(34-26)28(29)30/h3-5,8-9,11-12,14-15,18-19,27H,2,6-7,10,13,16-17H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor gamma using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168567
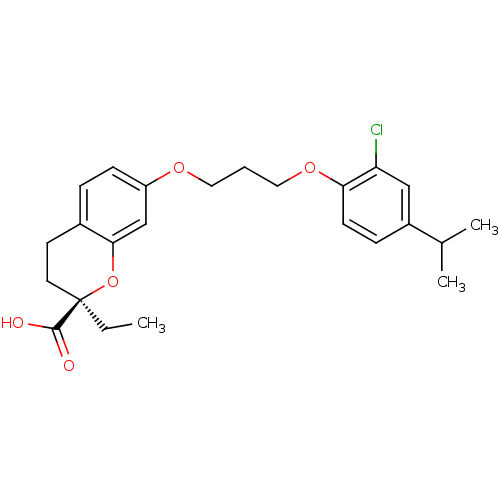
((R)-7-[3-(2-Chloro-4-isopropyl-phenoxy)-propoxy]-2...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C(C)C)cc2O1)C(O)=O Show InChI InChI=1S/C24H29ClO5/c1-4-24(23(26)27)11-10-17-6-8-19(15-22(17)30-24)28-12-5-13-29-21-9-7-18(16(2)3)14-20(21)25/h6-9,14-16H,4-5,10-13H2,1-3H3,(H,26,27)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50127922
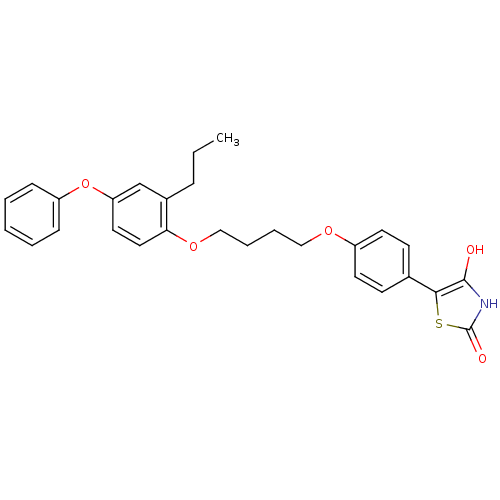
(5-{4-[4-(4-Phenoxy-2-propyl-phenoxy)-butoxy]-pheny...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCCOc1ccc(cc1)-c1sc(=O)[nH]c1O Show InChI InChI=1S/C28H29NO5S/c1-2-8-21-19-24(34-23-9-4-3-5-10-23)15-16-25(21)33-18-7-6-17-32-22-13-11-20(12-14-22)26-27(30)29-28(31)35-26/h3-5,9-16,19,30H,2,6-8,17-18H2,1H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPAR gamma (peroxisome proliferator-activated gamma receptor) |
Bioorg Med Chem Lett 13: 1801-4 (2003)
BindingDB Entry DOI: 10.7270/Q2H994K2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168557

((R)-7-[3-(2-Chloro-4-isobutyl-phenoxy)-propoxy]-2-...)Show SMILES CC(C)Cc1ccc(OCCCOc2ccc3CC[C@@](C)(Oc3c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C24H29ClO5/c1-16(2)13-17-5-8-21(20(25)14-17)29-12-4-11-28-19-7-6-18-9-10-24(3,23(26)27)30-22(18)15-19/h5-8,14-16H,4,9-13H2,1-3H3,(H,26,27)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50148091
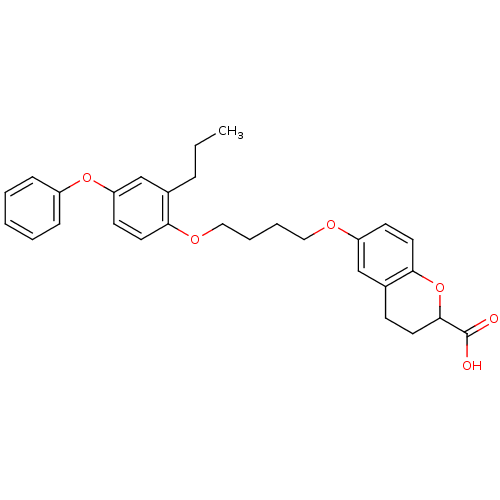
(6-[4-(4-Phenoxy-2-propyl-phenoxy)-butoxy]-chroman-...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCCOc1ccc2OC(CCc2c1)C(O)=O Show InChI InChI=1S/C29H32O6/c1-2-8-21-20-25(34-23-9-4-3-5-10-23)13-15-26(21)33-18-7-6-17-32-24-12-16-27-22(19-24)11-14-28(35-27)29(30)31/h3-5,9-10,12-13,15-16,19-20,28H,2,6-8,11,14,17-18H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor gamma using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148100

(2-tert-Butyl-7-[3-(4-phenoxy-2-propyl-phenoxy)-pro...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc2CCC(CC(C)C)(Oc2c1)C(O)=O Show InChI InChI=1S/C32H38O6/c1-4-9-25-20-28(37-26-10-6-5-7-11-26)14-15-29(25)36-19-8-18-35-27-13-12-24-16-17-32(31(33)34,22-23(2)3)38-30(24)21-27/h5-7,10-15,20-21,23H,4,8-9,16-19,22H2,1-3H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148101

(2-Methyl-6-[3-(4-phenoxy-2-propyl-phenoxy)-propoxy...)Show SMILES CCCc1cc(Oc2ccccc2)ccc1OCCCOc1ccc2OC(C)(CCc2c1)C(O)=O Show InChI InChI=1S/C29H32O6/c1-3-8-21-20-25(34-23-9-5-4-6-10-23)12-13-26(21)33-18-7-17-32-24-11-14-27-22(19-24)15-16-29(2,35-27)28(30)31/h4-6,9-14,19-20H,3,7-8,15-18H2,1-2H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168549
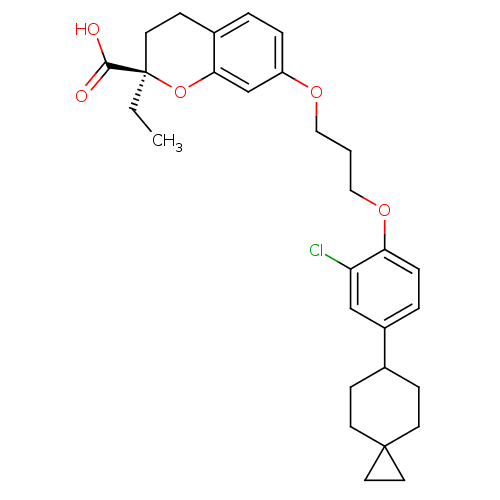
((R)-7-[3-(2-Chloro-4-spiro[2.5]oct-6-yl-phenoxy)-p...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C3CCC4(CC4)CC3)cc2O1)C(O)=O Show InChI InChI=1S/C29H35ClO5/c1-2-29(27(31)32)13-10-21-4-6-23(19-26(21)35-29)33-16-3-17-34-25-7-5-22(18-24(25)30)20-8-11-28(12-9-20)14-15-28/h4-7,18-20H,2-3,8-17H2,1H3,(H,31,32)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50168560

((R)-7-[3-(2-Chloro-4-cyclopentyl-phenoxy)-propoxy]...)Show SMILES C[C@@]1(CCc2ccc(OCCCOc3ccc(cc3Cl)C3CCCC3)cc2O1)C(O)=O Show InChI InChI=1S/C25H29ClO5/c1-25(24(27)28)12-11-18-7-9-20(16-23(18)31-25)29-13-4-14-30-22-10-8-19(15-21(22)26)17-5-2-3-6-17/h7-10,15-17H,2-6,11-14H2,1H3,(H,27,28)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARgamma |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50168562
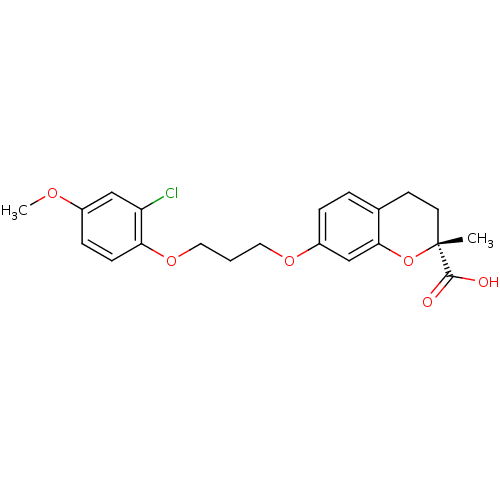
((R)-7-[3-(2-Chloro-4-methoxy-phenoxy)-propoxy]-2-m...)Show SMILES COc1ccc(OCCCOc2ccc3CC[C@@](C)(Oc3c2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C21H23ClO6/c1-21(20(23)24)9-8-14-4-5-16(13-19(14)28-21)26-10-3-11-27-18-7-6-15(25-2)12-17(18)22/h4-7,12-13H,3,8-11H2,1-2H3,(H,23,24)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARalpha |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50148102

(7-{3-[4-(4-Chloro-phenoxy)-2-propyl-phenoxy]-propo...)Show SMILES CCCc1cc(Oc2ccc(Cl)cc2)ccc1OCCCOc1ccc2CCC(CC)(Oc2c1)C(O)=O Show InChI InChI=1S/C30H33ClO6/c1-3-6-22-19-26(36-24-11-8-23(31)9-12-24)13-14-27(22)35-18-5-17-34-25-10-7-21-15-16-30(4-2,29(32)33)37-28(21)20-25/h7-14,19-20H,3-6,15-18H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human peroxisome proliferator activated receptor alpha using scintillation proximity assay (SPA) |
J Med Chem 47: 3255-63 (2004)
Article DOI: 10.1021/jm030621d
BindingDB Entry DOI: 10.7270/Q2JW8DB1 |
More data for this
Ligand-Target Pair | |
3-oxoacyl-[acyl-carrier-protein] synthase 3
(Mycobacterium tuberculosis) | BDBM50241313
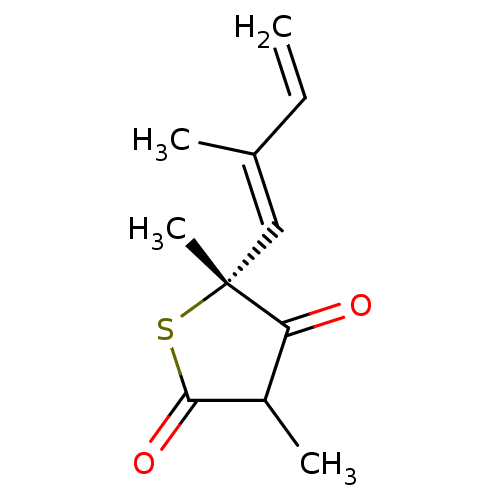
((5R)-4-hydroxy-3,5-dimethyl-5-[(1E)-2-methylbuta-1...)Show InChI InChI=1S/C11H14O2S/c1-5-7(2)6-11(4)9(12)8(3)10(13)14-11/h5-6,8H,1H2,2-4H3/b7-6+/t8?,11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases
Curated by ChEMBL
| Assay Description
Inhibitory activity against KasA from Mycobacterium tuberculosis H37Rv |
J Med Chem 49: 159-71 (2006)
Article DOI: 10.1021/jm050825p
BindingDB Entry DOI: 10.7270/Q2NG4RDB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50504041

(CHEMBL4529787)Show SMILES CC(C)[C@@H]1CC[C@H](C(O)=O)[C@]11CC=C(C)[C@@H](O)C1 |r,t:13| Show InChI InChI=1S/C15H24O3/c1-9(2)11-4-5-12(14(17)18)15(11)7-6-10(3)13(16)8-15/h6,9,11-13,16H,4-5,7-8H2,1-3H3,(H,17,18)/t11-,12+,13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal MKHQHQHQHQHQHQQPL-tagged 11beta-HSD1 C272S mutant (unknown origin) expressed in Escherichia coli BL21 (DE3) using cortisol a... |
J Med Chem 62: 6925-6940 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00187
BindingDB Entry DOI: 10.7270/Q23R0X43 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50168559

((R)-7-[3-(2,4-Dichloro-phenoxy)-propoxy]-2-ethyl-c...)Show SMILES CC[C@@]1(CCc2ccc(OCCCOc3ccc(Cl)cc3Cl)cc2O1)C(O)=O Show InChI InChI=1S/C21H22Cl2O5/c1-2-21(20(24)25)9-8-14-4-6-16(13-19(14)28-21)26-10-3-11-27-18-7-5-15(22)12-17(18)23/h4-7,12-13H,2-3,8-11H2,1H3,(H,24,25)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human PPARdelta |
Bioorg Med Chem Lett 15: 3347-51 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.028
BindingDB Entry DOI: 10.7270/Q22N51TV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data