 Found 294 hits Enz. Inhib. hit(s) with all data for entry = 50042808
Found 294 hits Enz. Inhib. hit(s) with all data for entry = 50042808 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine protease 1
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM639340
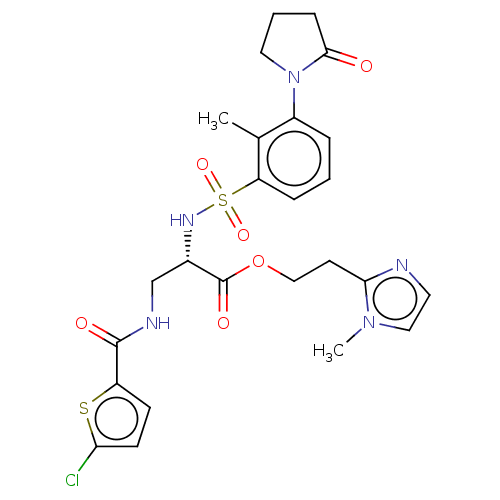
(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...)Show SMILES Cc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50547381
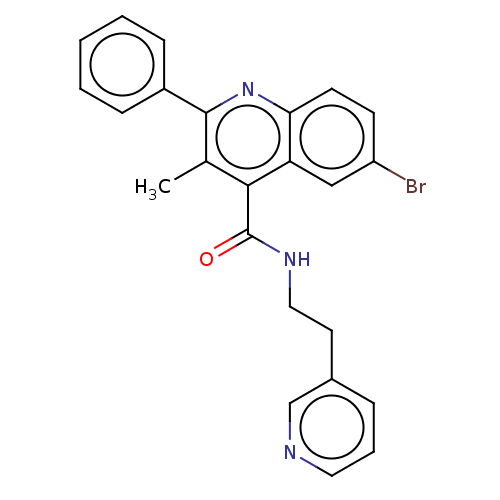
(CHEMBL4748513)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NCCc1cccnc1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human FPR expressed in human Chem-1 cells assessed as inhibition of PGF2alpha-induced calcium flux preincubated for 10 mins fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM639345

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES Cc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM639345

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES Cc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM639347
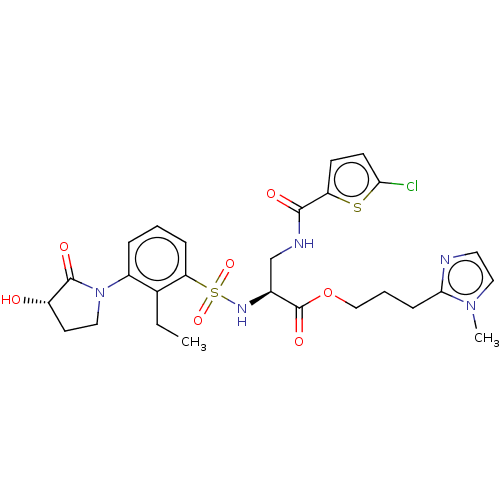
(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM639342
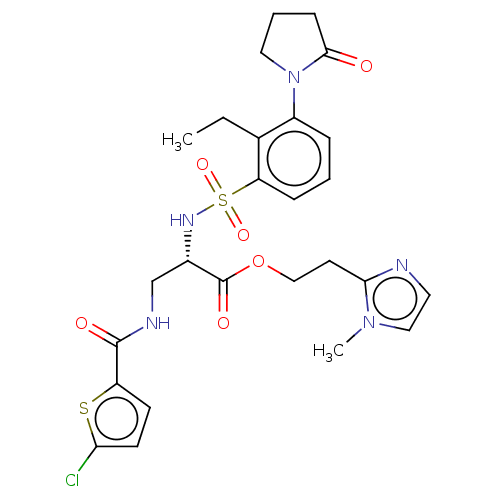
(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM639348
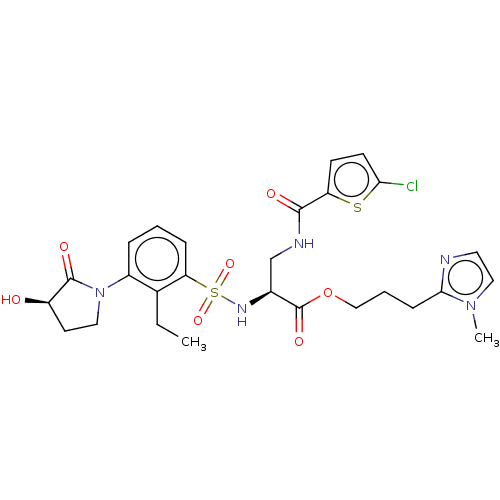
(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50547380
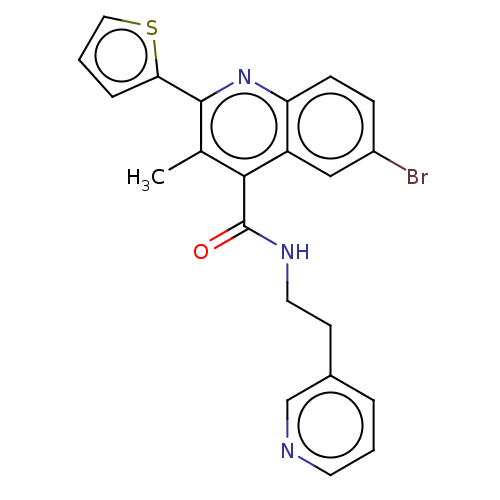
(CHEMBL4790821)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NCCc1cccnc1)-c1cccs1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]prostaglandin E2 from full-length recombinant human EP2 receptor expressed in HEK293 cell membranes measured after 120 mins by sc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM639343

(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520770
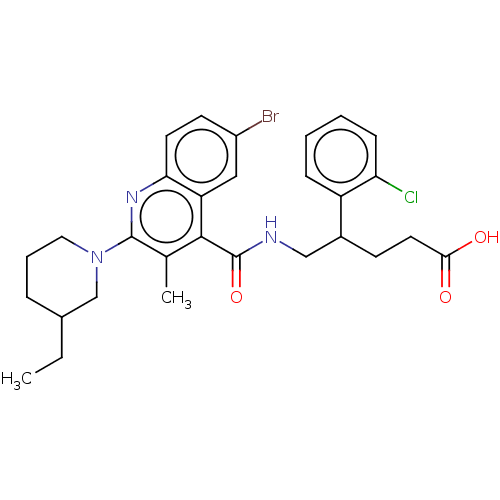
((+/−)-5-[({6-Bromo-2-[3-ethylpiperidin-1-yl]...)Show SMILES CCC1CCCN(C1)c1nc2ccc(Br)cc2c(C(=O)NCC(CCC(O)=O)c2ccccc2Cl)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520770

((+/−)-5-[({6-Bromo-2-[3-ethylpiperidin-1-yl]...)Show SMILES CCC1CCCN(C1)c1nc2ccc(Br)cc2c(C(=O)NCC(CCC(O)=O)c2ccccc2Cl)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520770

((+/−)-5-[({6-Bromo-2-[3-ethylpiperidin-1-yl]...)Show SMILES CCC1CCCN(C1)c1nc2ccc(Br)cc2c(C(=O)NCC(CCC(O)=O)c2ccccc2Cl)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50547377
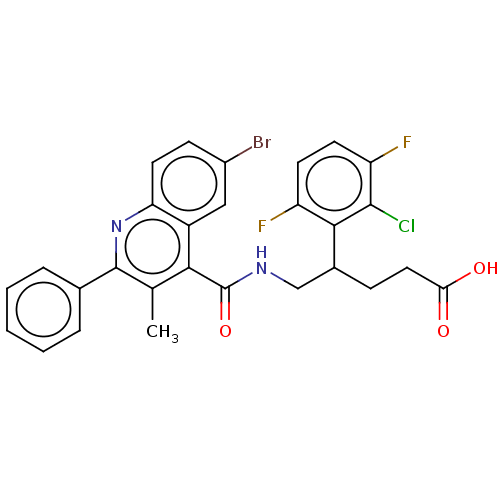
(CHEMBL4749767)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NCC(CCC(O)=O)c1c(F)ccc(F)c1Cl)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM639321

(Methyl 3-{[(5-chloro-2-thienyl)carbonyl]amino}-N-{...)Show SMILES COC(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50547364
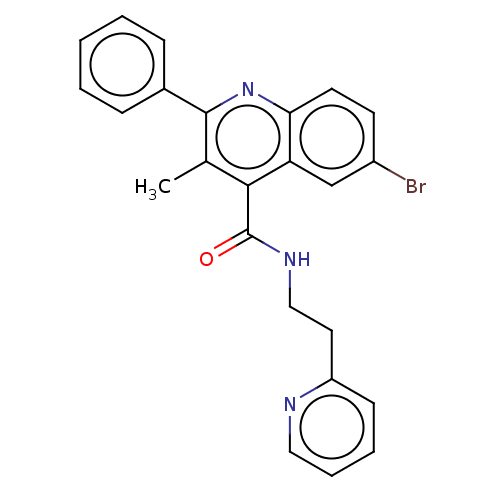
(CHEMBL4778430)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NCCc1ccccn1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM639343

(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM639341
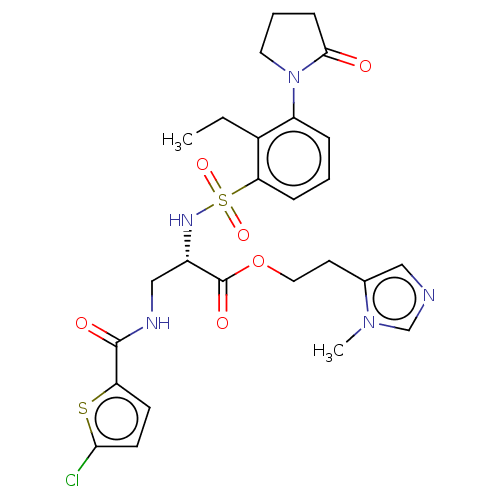
(2-(1-Methyl-1H-imidazol-5-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1cncn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM639349

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM639346

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM639320
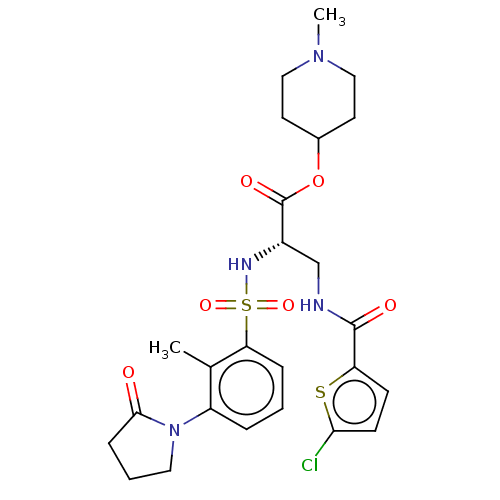
(1-Methylpiperidin-4-yl 3-{[(5-chloro-2-thienyl)car...)Show SMILES CN1CCC(CC1)OC(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM639341

(2-(1-Methyl-1H-imidazol-5-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1cncn1C)N1CCCC1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM639348

(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM639343

(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM639347

(3-(1-Methyl-1H-imidazol-2-yl)propyl 3-[(5-chloroth...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCCc1nccn1C)N1CC[C@H](O)C1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50547379
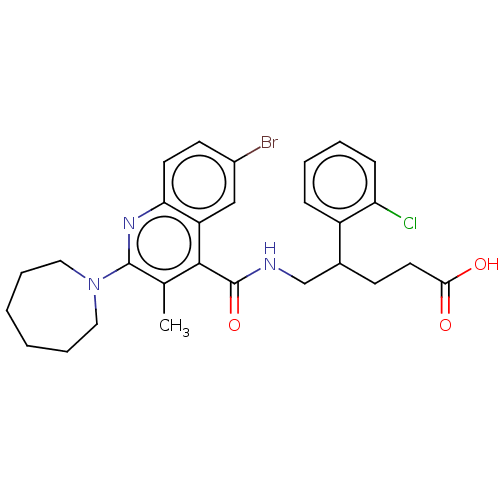
(CHEMBL4784864)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NCC(CCC(O)=O)c1ccccc1Cl)N1CCCCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50547367
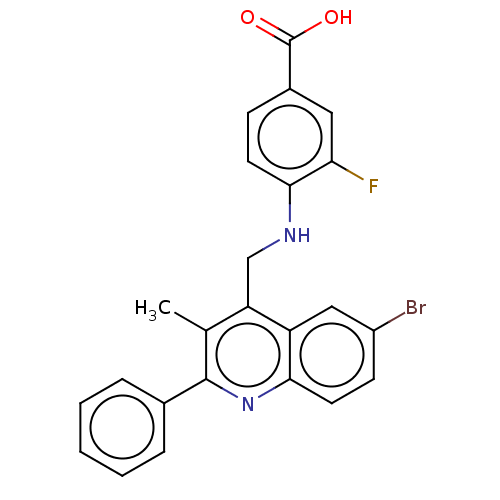
(CHEMBL4756085)Show SMILES Cc1c(CNc2ccc(cc2F)C(O)=O)c2cc(Br)ccc2nc1-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50547379

(CHEMBL4784864)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NCC(CCC(O)=O)c1ccccc1Cl)N1CCCCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM639340

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...)Show SMILES Cc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM639342

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM520995
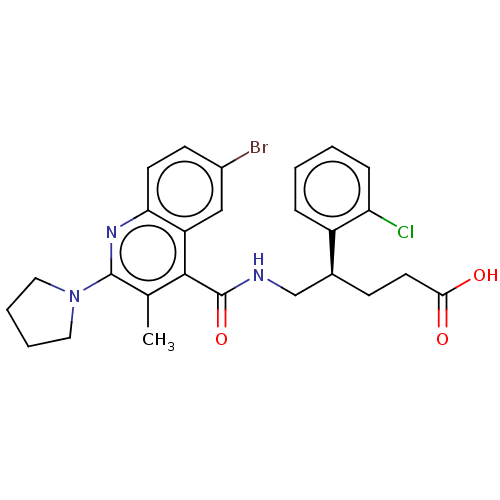
((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC[C@H](CCC(O)=O)c1ccccc1Cl)N1CCCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]prostaglandin E2 from full-length recombinant human EP4 receptor expressed in HEK293 cell membranes measured after 120 mins by sc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM520995

((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC[C@H](CCC(O)=O)c1ccccc1Cl)N1CCCC1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]prostaglandin E2 from full-length recombinant human EP3 receptor expressed in HEK293 cell membranes measured after 120 mins by sc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM520995

((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC[C@H](CCC(O)=O)c1ccccc1Cl)N1CCCC1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGD2 from full-length recombinant human IP receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520770

((+/−)-5-[({6-Bromo-2-[3-ethylpiperidin-1-yl]...)Show SMILES CCC1CCCN(C1)c1nc2ccc(Br)cc2c(C(=O)NCC(CCC(O)=O)c2ccccc2Cl)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM297411
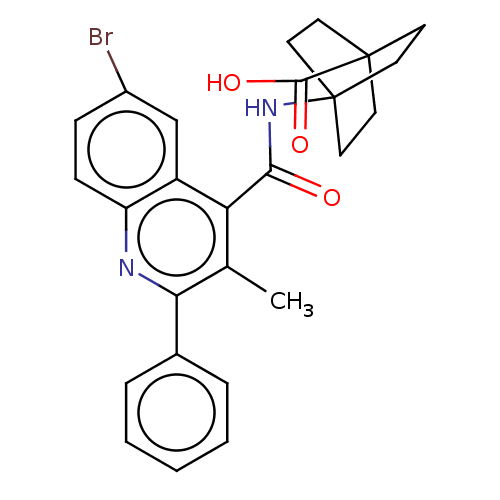
(4-{[(6-Bromo-3-methyl-2-phenylquinolin-4-yl)carbon...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC12CCC(CC1)(CC2)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C26H25BrN2O3/c1-16-21(23(30)29-26-12-9-25(10-13-26,11-14-26)24(31)32)19-15-18(27)7-8-20(19)28-22(16)17-5-3-2-4-6-17/h2-8,15H,9-14H2,1H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate incubated for 15 mins in presence of NADPH generating system by LC-MS/MS... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM297411

(4-{[(6-Bromo-3-methyl-2-phenylquinolin-4-yl)carbon...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC12CCC(CC1)(CC2)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C26H25BrN2O3/c1-16-21(23(30)29-26-12-9-25(10-13-26,11-14-26)24(31)32)19-15-18(27)7-8-20(19)28-22(16)17-5-3-2-4-6-17/h2-8,15H,9-14H2,1H3,(H,29,30)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate incubated for 15 mins in presence of NADPH generating system by LC-MS/M... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM520995

((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC[C@H](CCC(O)=O)c1ccccc1Cl)N1CCCC1 |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGD2 from full-length recombinant human DP1 receptor expressed in Chem-1 cell membranes measured after 120 mins by scintillation ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM520995

((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC[C@H](CCC(O)=O)c1ccccc1Cl)N1CCCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PGD2 from full-length recombinant human CRTH2 receptor expressed in CHOK1 cell membranes measured after 120 mins by scintillation... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM520995

((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC[C@H](CCC(O)=O)c1ccccc1Cl)N1CCCC1 |r| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]prostaglandin E2 from full-length recombinant human EP1 receptor expressed in HEK293 cell membranes measured after 60 mins by sci... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM520995

((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC[C@H](CCC(O)=O)c1ccccc1Cl)N1CCCC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]prostaglandin E2 from full-length recombinant human EP2 receptor expressed in HEK293 cell membranes measured after 120 mins by sc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM297411

(4-{[(6-Bromo-3-methyl-2-phenylquinolin-4-yl)carbon...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC12CCC(CC1)(CC2)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C26H25BrN2O3/c1-16-21(23(30)29-26-12-9-25(10-13-26,11-14-26)24(31)32)19-15-18(27)7-8-20(19)28-22(16)17-5-3-2-4-6-17/h2-8,15H,9-14H2,1H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM297411

(4-{[(6-Bromo-3-methyl-2-phenylquinolin-4-yl)carbon...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC12CCC(CC1)(CC2)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C26H25BrN2O3/c1-16-21(23(30)29-26-12-9-25(10-13-26,11-14-26)24(31)32)19-15-18(27)7-8-20(19)28-22(16)17-5-3-2-4-6-17/h2-8,15H,9-14H2,1H3,(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 15 mins in presence of NADPH generating system by LC... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM297411

(4-{[(6-Bromo-3-methyl-2-phenylquinolin-4-yl)carbon...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NC12CCC(CC1)(CC2)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C26H25BrN2O3/c1-16-21(23(30)29-26-12-9-25(10-13-26,11-14-26)24(31)32)19-15-18(27)7-8-20(19)28-22(16)17-5-3-2-4-6-17/h2-8,15H,9-14H2,1H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate incubated for 15 mins in presence of NADPH generating system by LC-MS/MS... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM639319
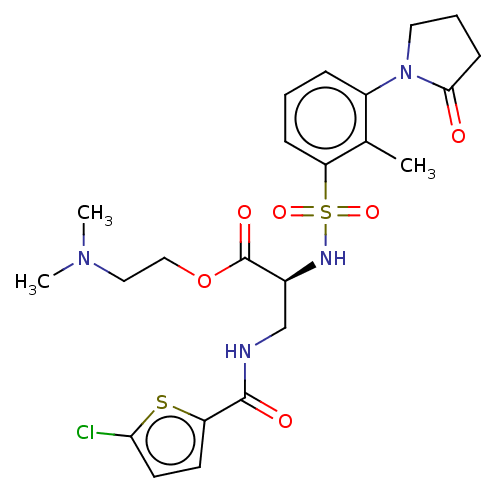
(2-(Dimethylamino)ethyl 3-{[(5-chloro-2-thienyl)car...)Show SMILES CN(C)CCOC(=O)[C@H](CNC(=O)c1ccc(Cl)s1)NS(=O)(=O)c1cccc(N2CCCC2=O)c1C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520867

((+/−)-5-({[6-Bromo-3-methyl-2-(piperidin-1-y...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NCC(CCC(O)=O)c1c(F)ccc(F)c1Cl)N1CCCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520867

((+/−)-5-({[6-Bromo-3-methyl-2-(piperidin-1-y...)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NCC(CCC(O)=O)c1c(F)ccc(F)c1Cl)N1CCCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM639346

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CC[C@@H](O)C1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM639342

(2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1nccn1C)N1CCCC1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50547372
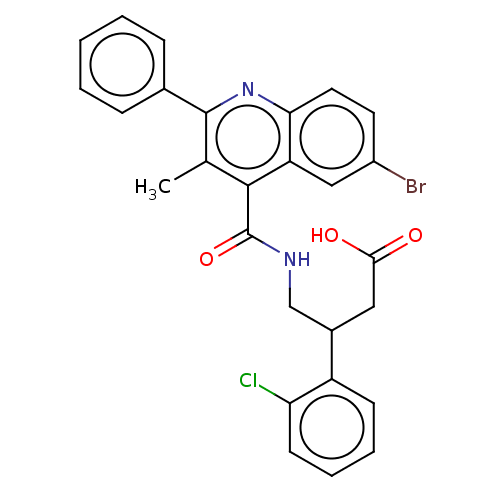
(CHEMBL4744552)Show SMILES Cc1c(nc2ccc(Br)cc2c1C(=O)NCC(CC(O)=O)c1ccccc1Cl)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH generating system by LC-MS/MS ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00834
BindingDB Entry DOI: 10.7270/Q2FJ2MC9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data