

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ubiquitin carboxyl-terminal hydrolase 5 (Homo sapiens (Human)) | BDBM50412346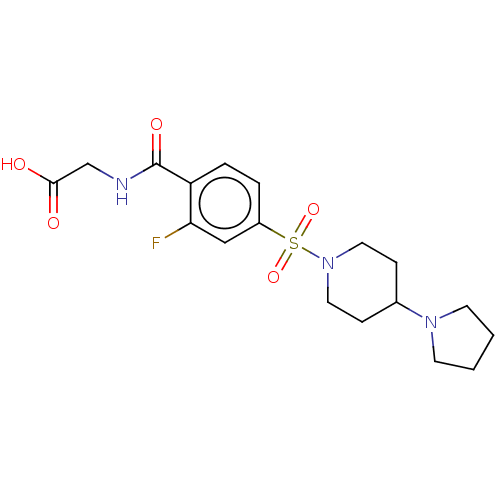 (CHEMBL5283754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Binding affinity to Human immunodeficiency virus 1 reverse transcriptase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50412346 (CHEMBL5283754) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of AChE in organophosphate-resistant clone of Schizaphis graminum OR2 adult or last-instar nymphs homogenates assessed as bimolecular rate... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||