

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM19856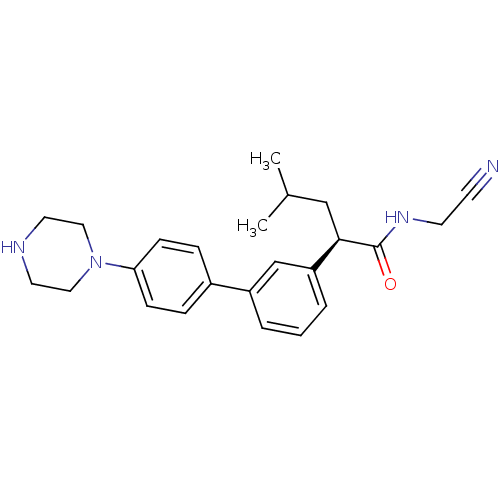 ((2R)-N-(cyanomethyl)-4-methyl-2-{3-[4-(piperazin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19885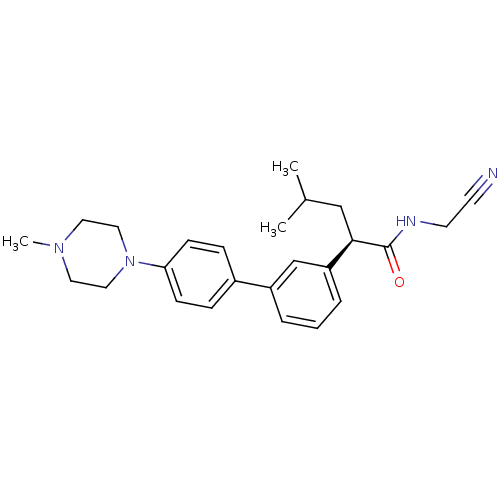 ((2R)-N-(cyanomethyl)-4-methyl-2-{3-[4-(4-methylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19893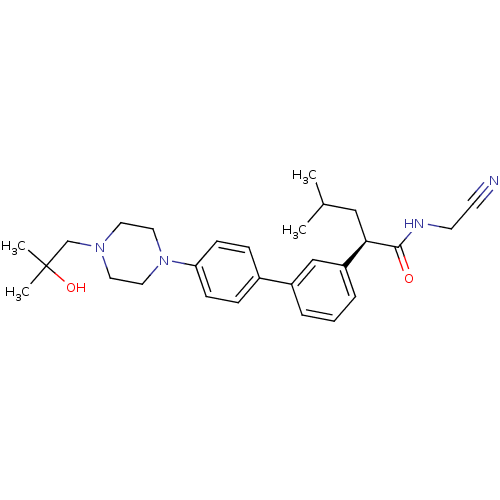 ((2R)-N-(cyanomethyl)-2-(3-{4-[4-(2-hydroxy-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19880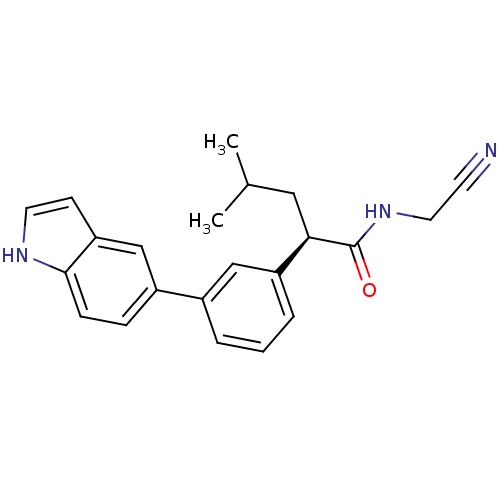 ((2R)-N-(cyanomethyl)-2-[3-(1H-indol-5-yl)phenyl]-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19884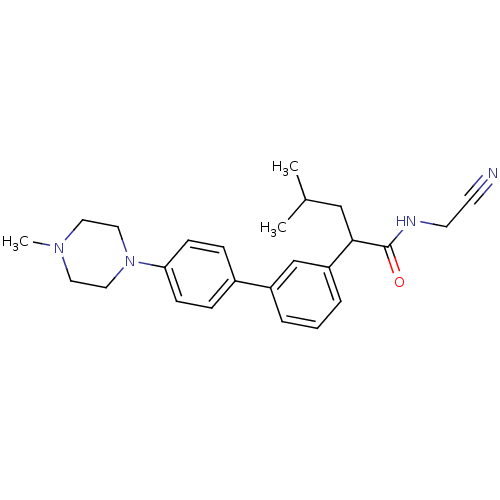 (N-(cyanomethyl)-4-methyl-2-{3-[4-(4-methylpiperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19883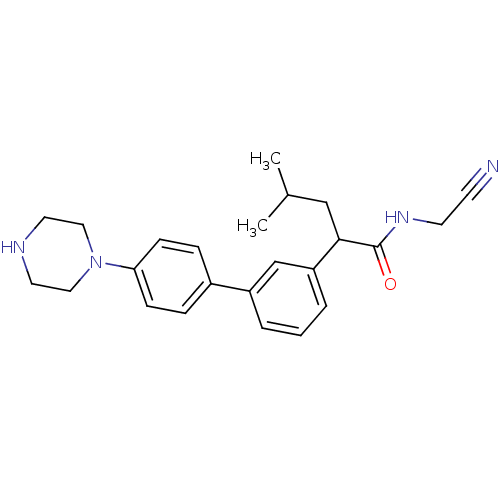 (N-(cyanomethyl)-4-methyl-2-{3-[4-(piperazin-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19890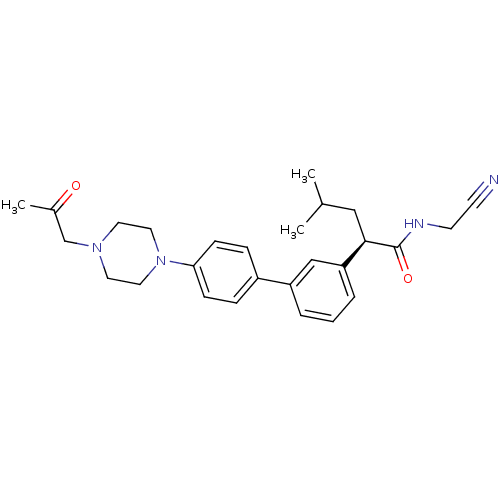 ((2R)-N-(cyanomethyl)-4-methyl-2-(3-{4-[4-(2-oxopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19892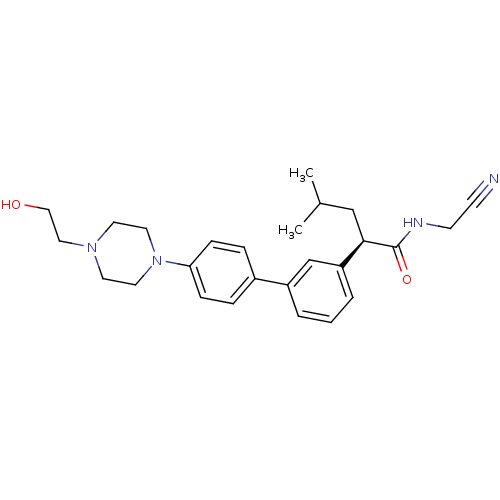 ((2R)-N-(cyanomethyl)-2-(3-{4-[4-(2-hydroxyethyl)pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19894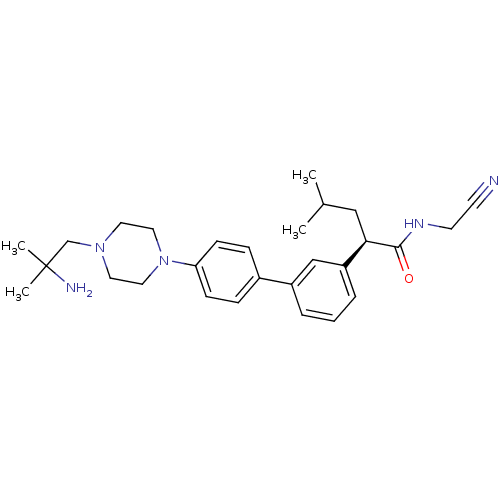 ((2R)-2-(3-{4-[4-(2-amino-2-methylpropyl)piperazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19887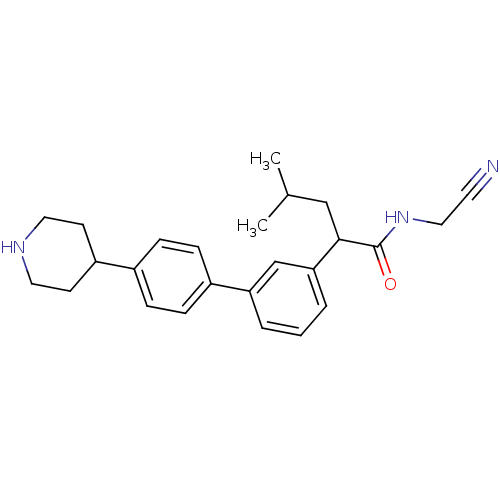 (N-(cyanomethyl)-4-methyl-2-{3-[4-(piperidin-4-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19891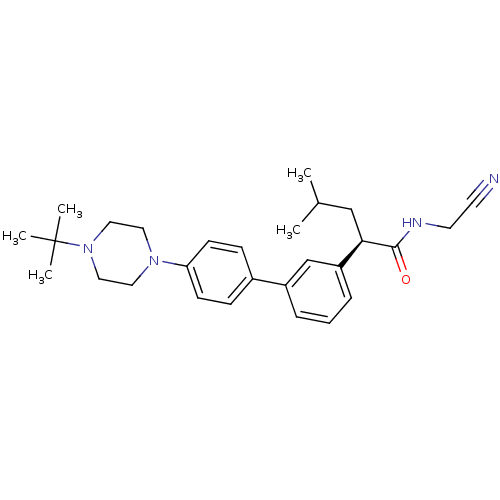 ((2R)-2-{3-[4-(4-tert-butylpiperazin-1-yl)phenyl]ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19888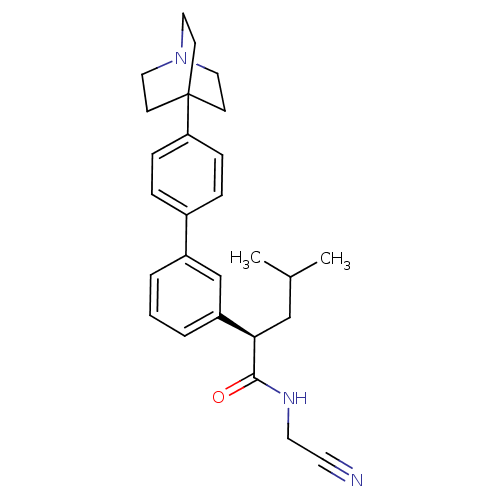 ((2R)-2-[3-(4-{1-azabicyclo[2.2.2]octan-4-yl}phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19861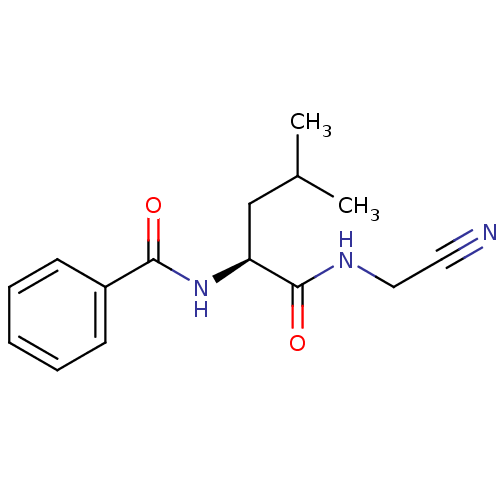 ((2S)-N-(cyanomethyl)-4-methyl-2-(phenylformamido)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19861 ((2S)-N-(cyanomethyl)-4-methyl-2-(phenylformamido)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19899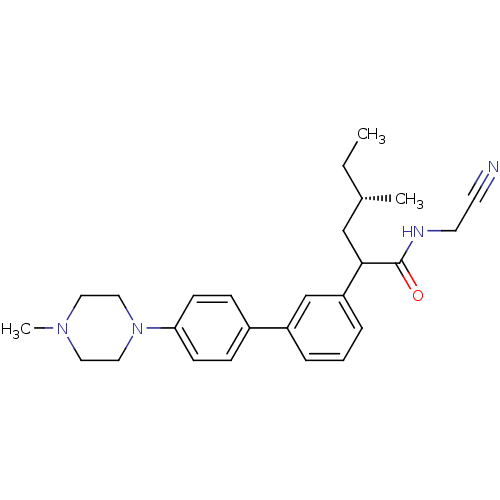 ((4S)-N-(cyanomethyl)-4-methyl-2-{3-[4-(4-methylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19861 ((2S)-N-(cyanomethyl)-4-methyl-2-(phenylformamido)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19878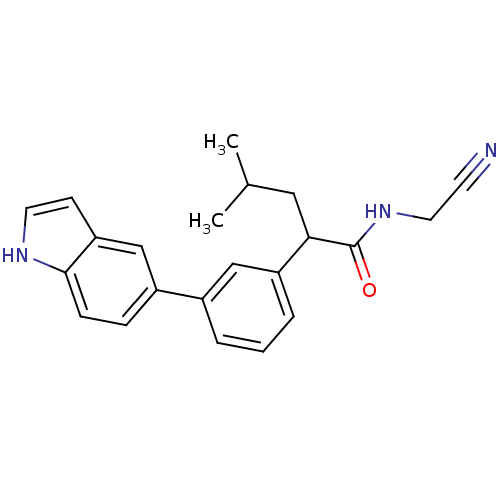 (N-(cyanomethyl)-2-[3-(1H-indol-5-yl)phenyl]-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19862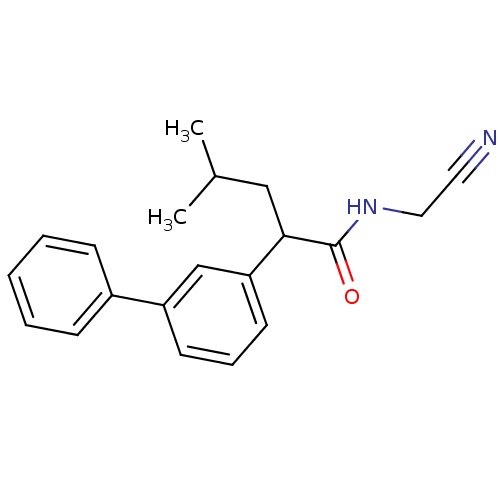 (N-(cyanomethyl)-4-methyl-2-(3-phenylphenyl)pentana...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19886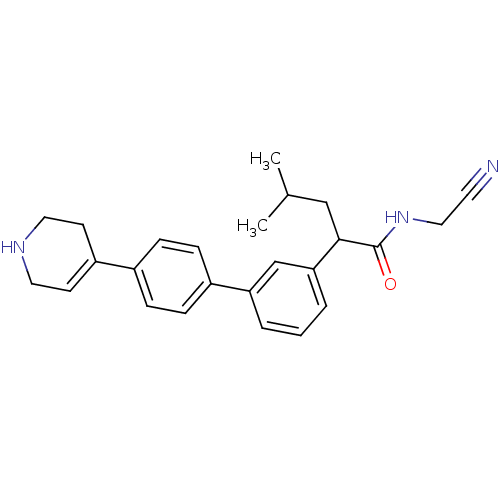 (N-(cyanomethyl)-4-methyl-2-{3-[4-(1,2,3,6-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19882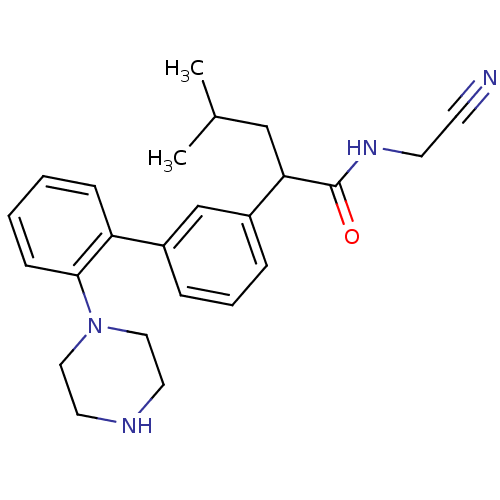 (N-(cyanomethyl)-4-methyl-2-{3-[2-(piperazin-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19900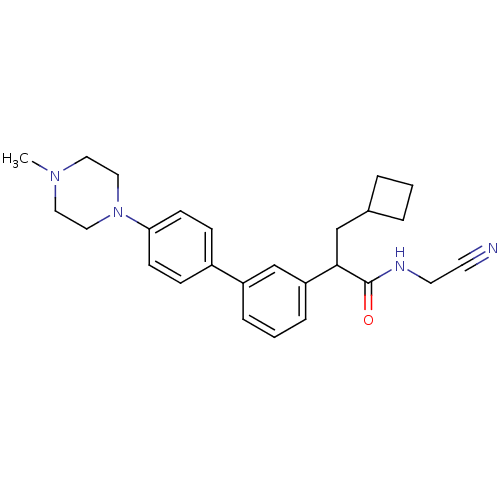 (N-(cyanomethyl)-3-cyclobutyl-2-{3-[4-(4-methylpipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19898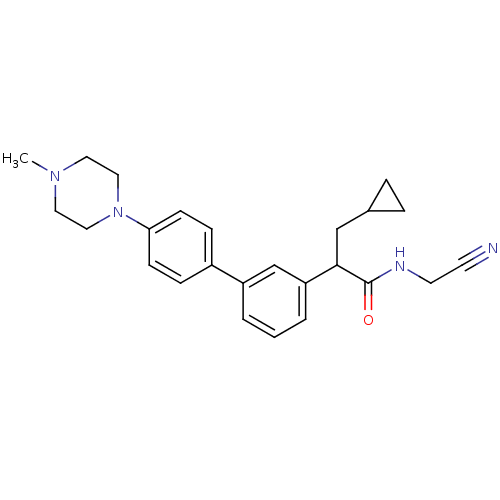 (N-(cyanomethyl)-3-cyclopropyl-2-{3-[4-(4-methylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19879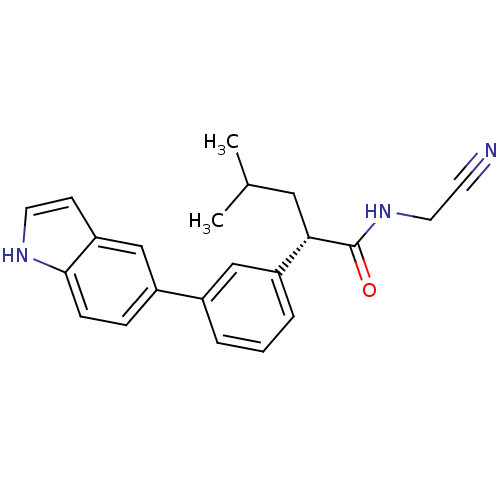 ((2S)-N-(cyanomethyl)-2-[3-(1H-indol-5-yl)phenyl]-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19897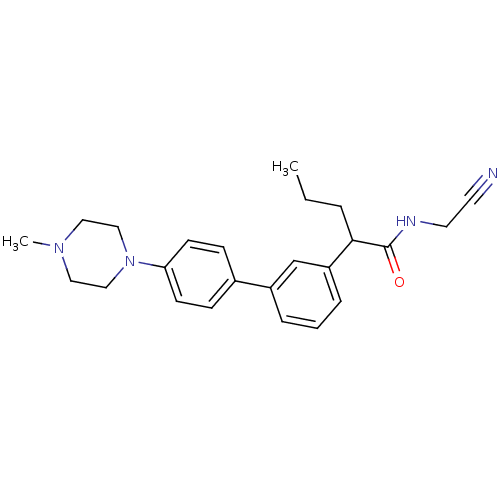 (N-(cyanomethyl)-2-{3-[4-(4-methylpiperazin-1-yl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19876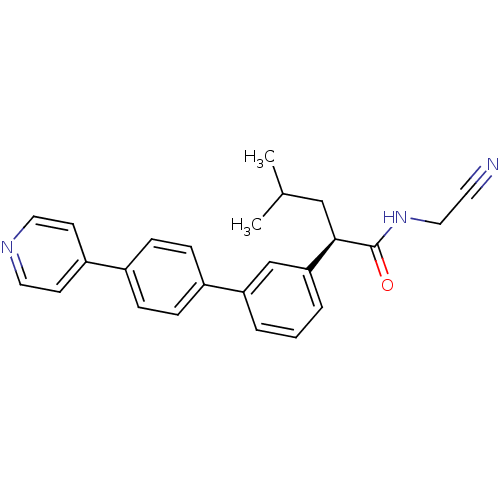 ((2R)-N-(Cyanomethyl)-4-methyl-2-[4 -(4-pyridinyl)[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19892 ((2R)-N-(cyanomethyl)-2-(3-{4-[4-(2-hydroxyethyl)pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19864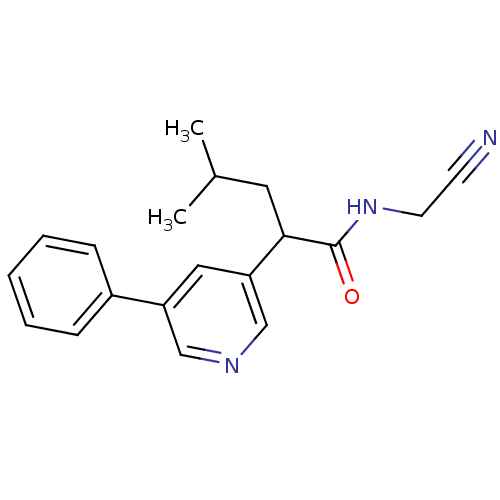 (N-(cyanomethyl)-4-methyl-2-(5-phenylpyridin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19875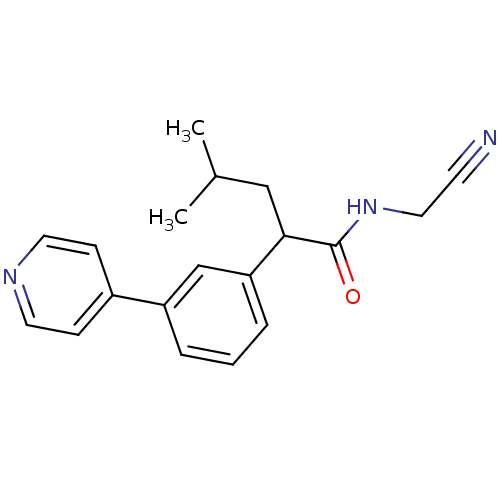 (N-(cyanomethyl)-4-methyl-2-[3-(pyridin-4-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19881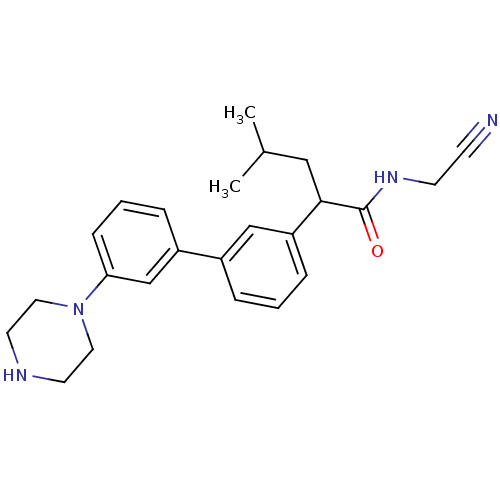 (N-(cyanomethyl)-4-methyl-2-{3-[3-(piperazin-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 216 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19877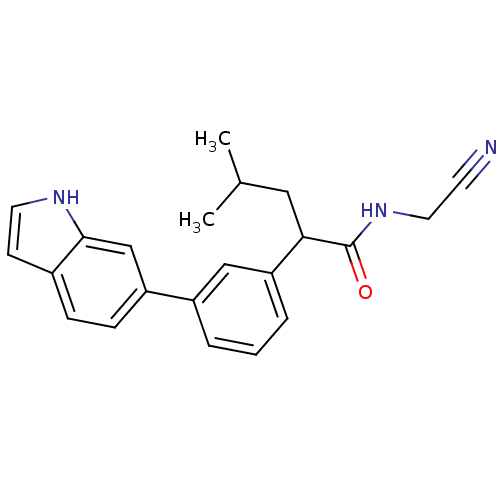 (N-(cyanomethyl)-2-[3-(1H-indol-6-yl)phenyl]-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 291 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19882 (N-(cyanomethyl)-4-methyl-2-{3-[2-(piperazin-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19891 ((2R)-2-{3-[4-(4-tert-butylpiperazin-1-yl)phenyl]ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19874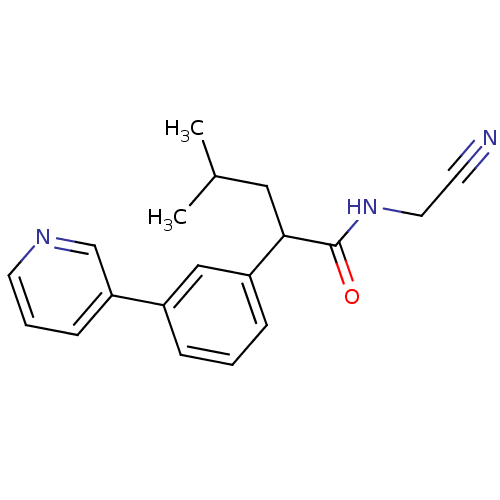 (N-(cyanomethyl)-4-methyl-2-[3-(pyridin-3-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 488 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19862 (N-(cyanomethyl)-4-methyl-2-(3-phenylphenyl)pentana...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 498 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19882 (N-(cyanomethyl)-4-methyl-2-{3-[2-(piperazin-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 521 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19890 ((2R)-N-(cyanomethyl)-4-methyl-2-(3-{4-[4-(2-oxopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19886 (N-(cyanomethyl)-4-methyl-2-{3-[4-(1,2,3,6-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19892 ((2R)-N-(cyanomethyl)-2-(3-{4-[4-(2-hydroxyethyl)pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 609 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19871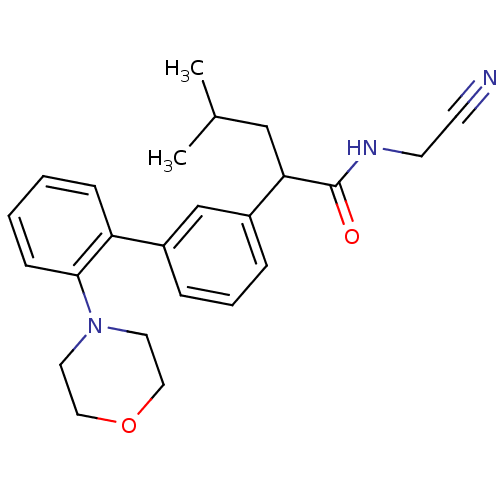 (N-(cyanomethyl)-4-methyl-2-{3-[2-(morpholin-4-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 614 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19870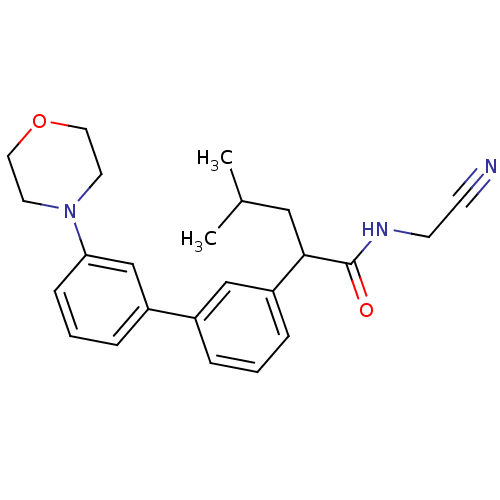 (N-(cyanomethyl)-4-methyl-2-{3-[3-(morpholin-4-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19869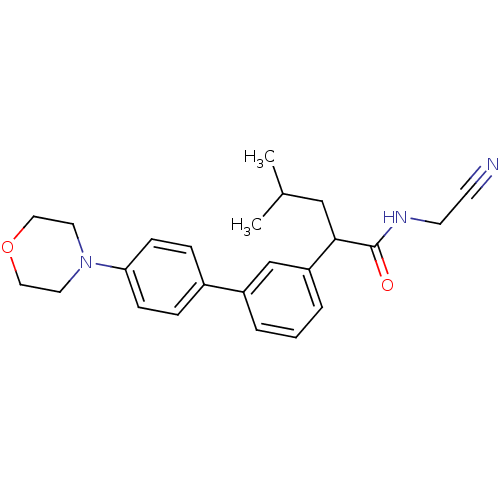 (N-(cyanomethyl)-4-methyl-2-{3-[4-(morpholin-4-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 638 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19886 (N-(cyanomethyl)-4-methyl-2-{3-[4-(1,2,3,6-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 652 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19863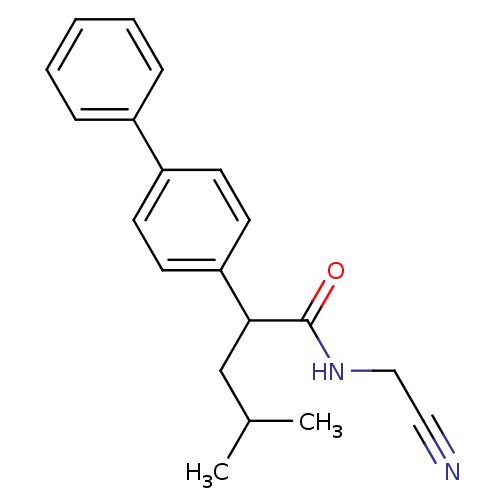 (N-(cyanomethyl)-4-methyl-2-(4-phenylphenyl)pentana...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19873 (N-(cyanomethyl)-4-methyl-2-[3-(pyridin-2-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 803 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19864 (N-(cyanomethyl)-4-methyl-2-(5-phenylpyridin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 825 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19871 (N-(cyanomethyl)-4-methyl-2-{3-[2-(morpholin-4-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 919 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19889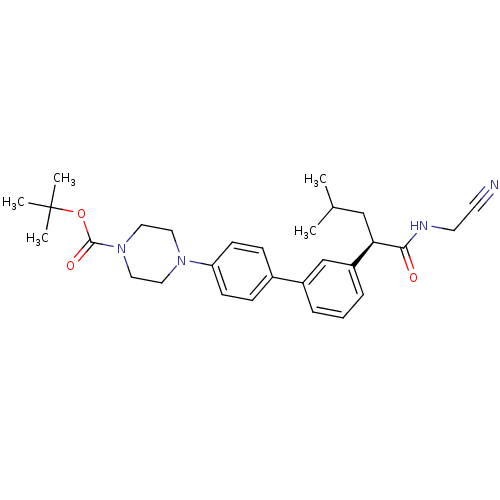 (Nonpeptidic Biaryl Nitrile Compound, 50 | tert-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 922 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19878 (N-(cyanomethyl)-2-[3-(1H-indol-5-yl)phenyl]-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 922 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19866 (4-(3-{1-[(cyanomethyl)carbamoyl]-3-methylbutyl}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19872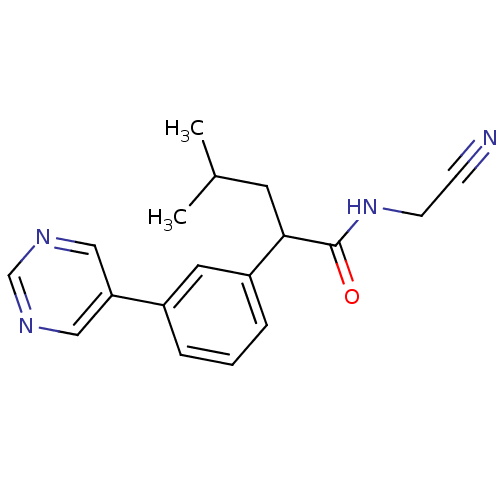 (N-(Cyanomethyl)-4-methyl-2-(3-pyrimidin-5-ylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 46: 3709-27 (2003) Article DOI: 10.1021/jm0301078 BindingDB Entry DOI: 10.7270/Q2513WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |