

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261893 (2-(6-Chloro-9-ethyl-9H-carbazol-3-yl)-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.16 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261894 (2-(8-Chloro-9-ethyl-9H-carbazol-3-yl)-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.19 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261752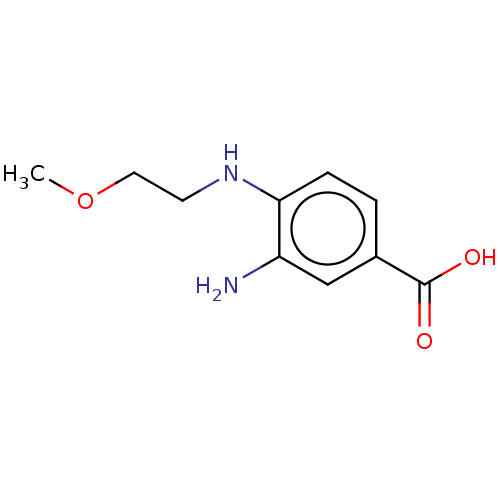 (3-Amino-4-[(2-methoxyethyl)amino]benzoic acid | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 5.59 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261760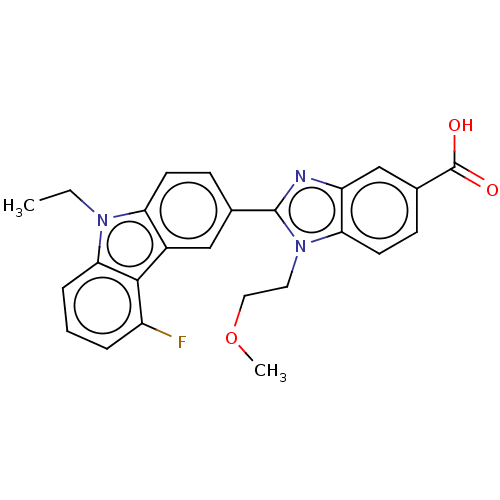 (2-(9-Ethyl-5-fluoro-9H-carbazol-3-yl)-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.36 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261864 (2-(9-Ethyl-9H-carbazol-3-yl)-4-fluoro-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.42 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261884 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(tetrahydrofuran-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261761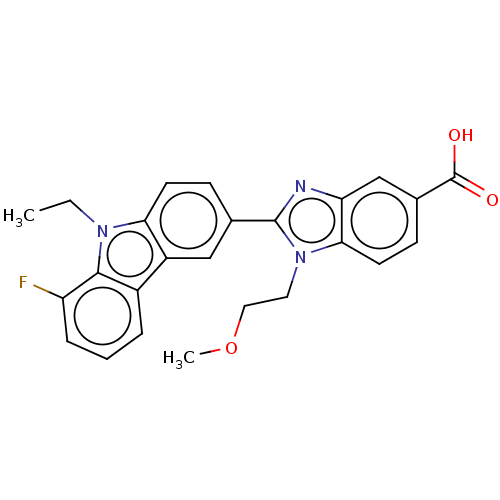 (2-(9-Ethyl-8-fluoro-9H-carbazol-3-yl)-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261759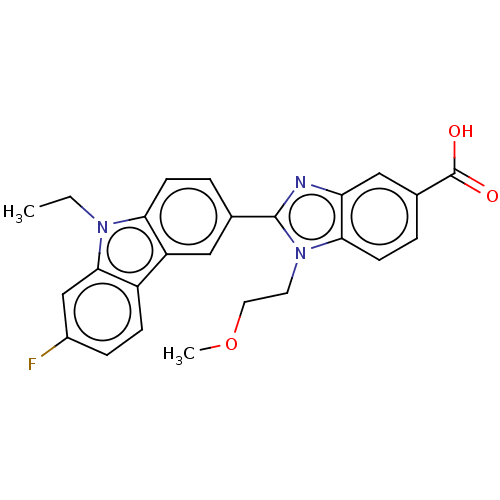 (2-(9-Ethyl-7-fluoro-9H-carbazol-3-yl)-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261865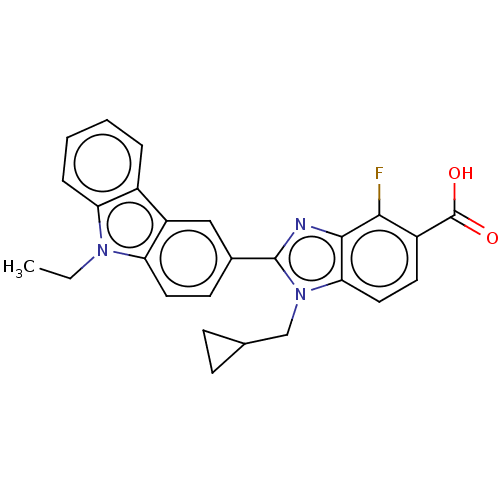 (1-(Cyclopropylmethyl)-2-(9-ethyl-9H-carbazol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261795 (3-[2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261785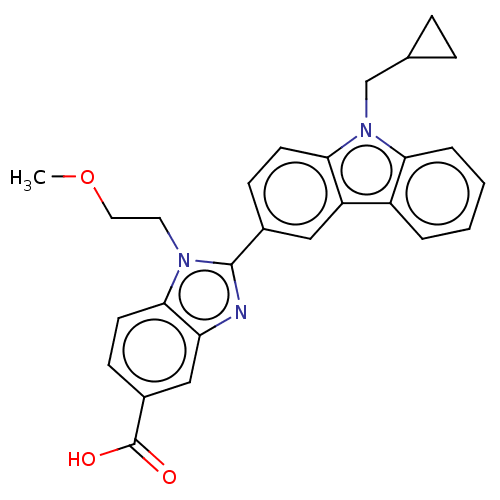 (2-[9-(Cyclopropylmethyl)-9H-carbazol-3-yl]-1-(2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261794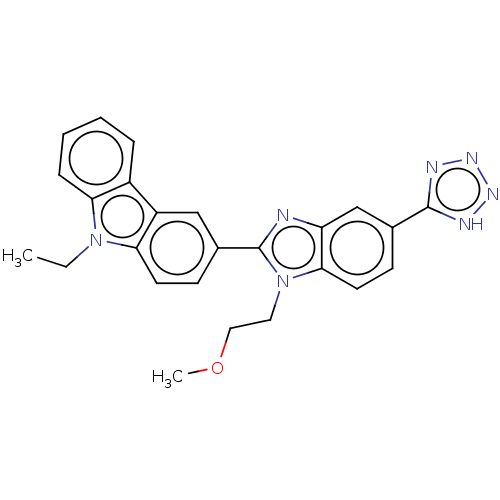 (9-Ethyl-3-[1-(2-methoxyethyl)-5-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261881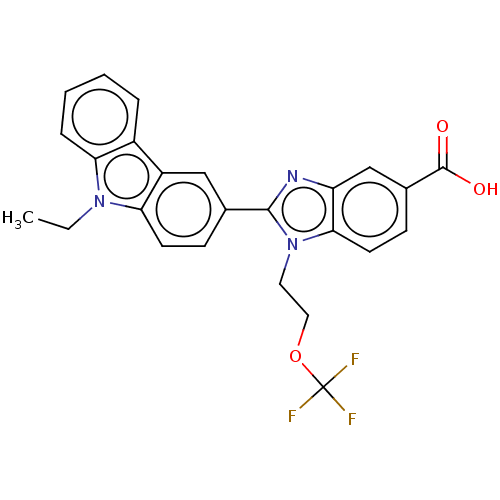 (2-(9-Ethyl-9H-carbazol-3-yl)-1-[2-(trifluoromethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261754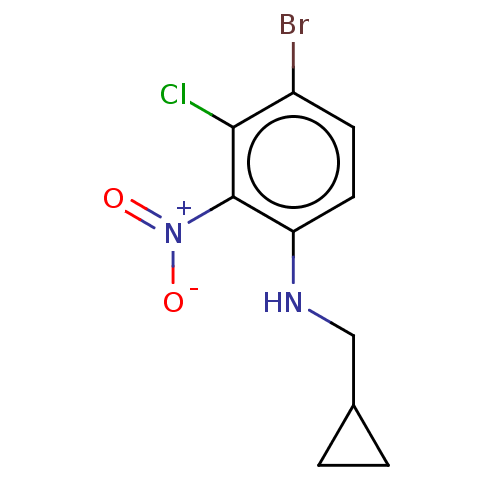 (4-Bromo-3-chloro-N-(cyclopropylmethyl)-2-nitroanil...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261766 (N-[(3-Chlorophenyl)sulphonyl]-2-(9-ethyl-9H-carbaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.7 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261892 (2-(9-Ethyl-6-methyl-9H-carbazol-3-yl)-1-(2-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261815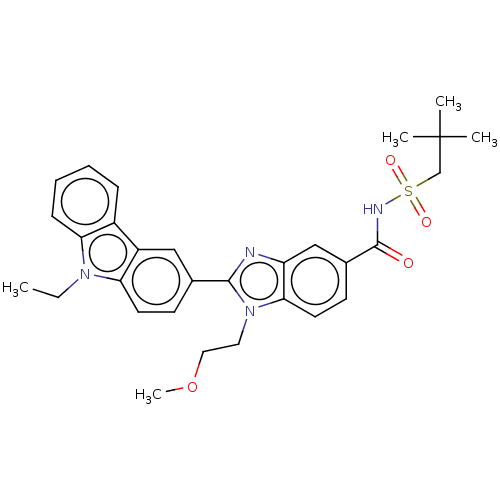 (N-[(2,2-Dimethylpropyl)sulphonyl]-2-(9-ethyl-9H-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.1 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261846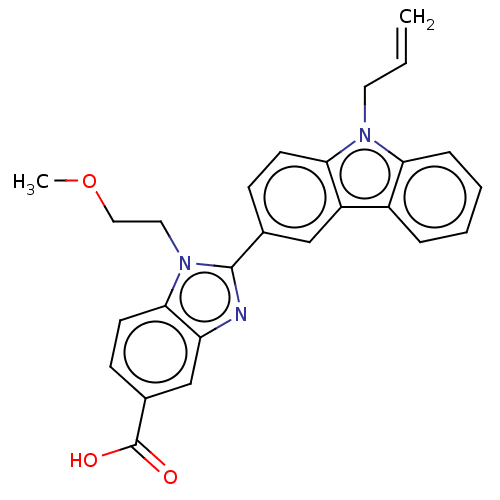 (2-(9-Allyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.5 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261808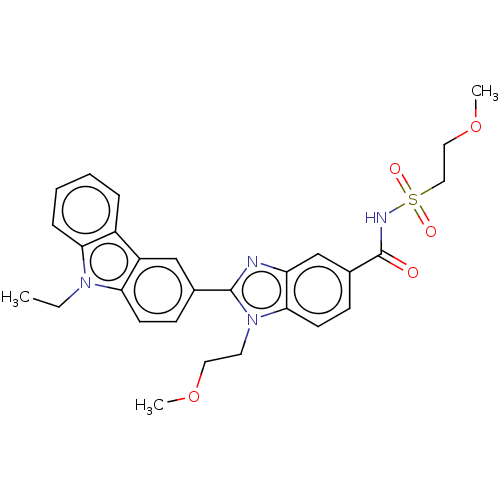 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261803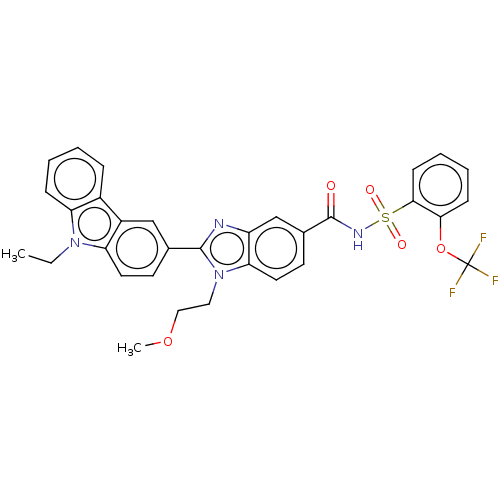 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261762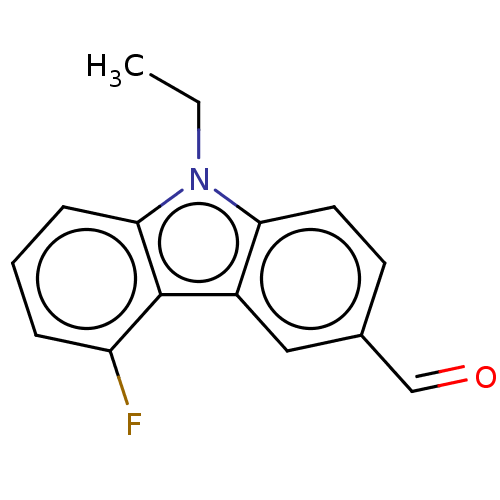 (9-Ethyl-5-fluoro-9H-carbazole-3-carbaldehyde | US9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261888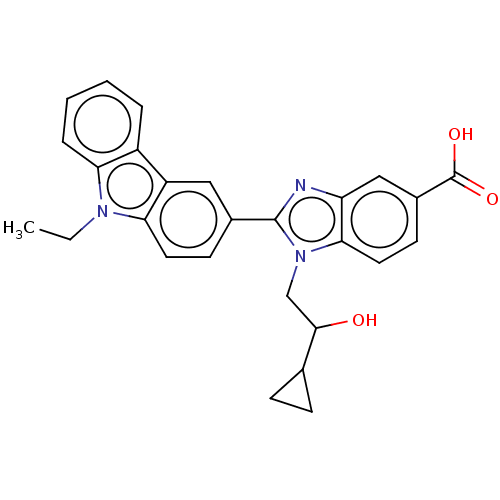 (1-(2-Cyclopropyl-2-hydroxyethyl)-2-(9-ethyl-9H-car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261756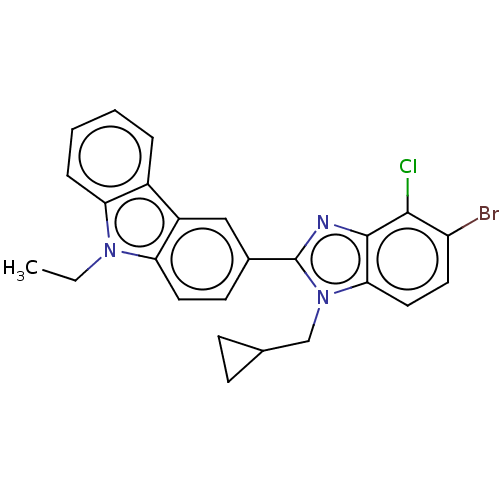 (3-[5-Bromo-4-chloro-1-(cyclopropylmethyl)-1H-benzi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261809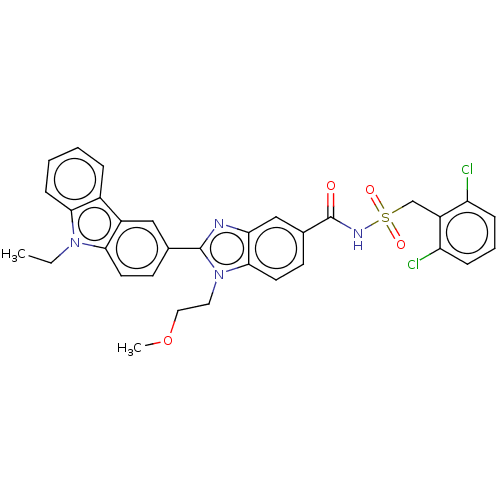 (N-[(2,6-Dichlorbenzyl)sulphonyl]-2-(9-ethyl-9H-car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.8 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261824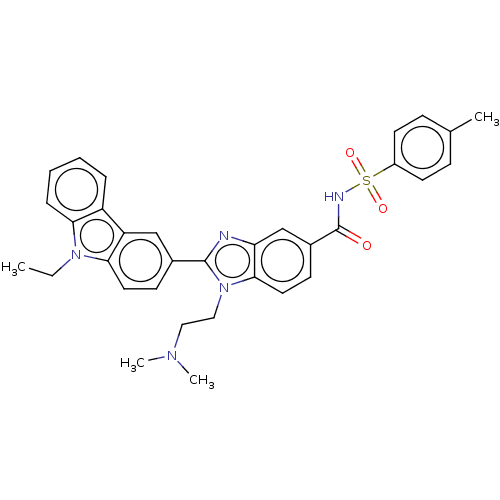 (1-[2-(Dimethylamino)ethyl]-2-(9-ethyl-9H-carbazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.4 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261867 (1-(2-Cyclopropylethyl)-2-(9-ethyl-9H-carbazol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.1 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261866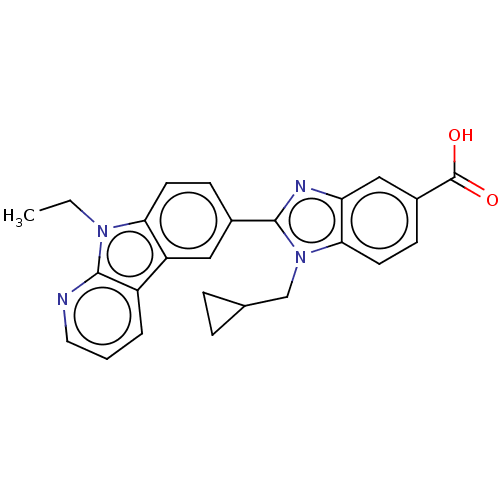 (1-(Cyclopropylmethyl)-2-(9-ethyl-9H-pyrido[2,3-b]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.2 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261767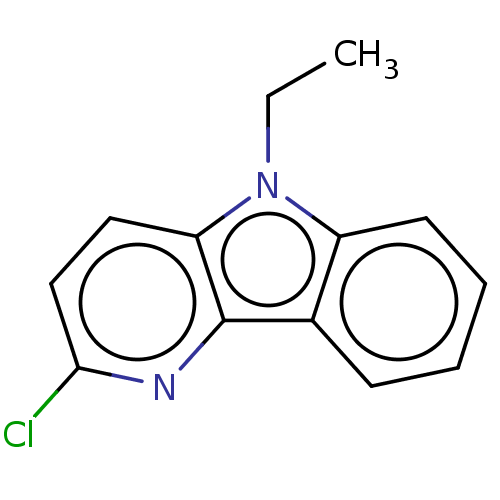 (2-Chloro-5-ethyl-5H-pyrido[3,2-b]indole | US970831...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.8 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261764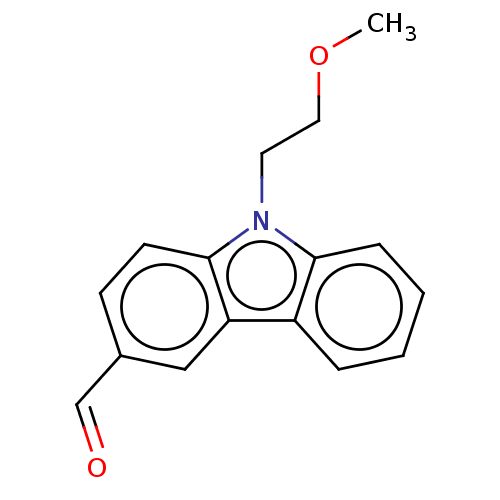 (9-(2-Methoxyethyl)-9H-carbazole-3-carbaldehyde | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23.8 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261883 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(oxetan-2-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261796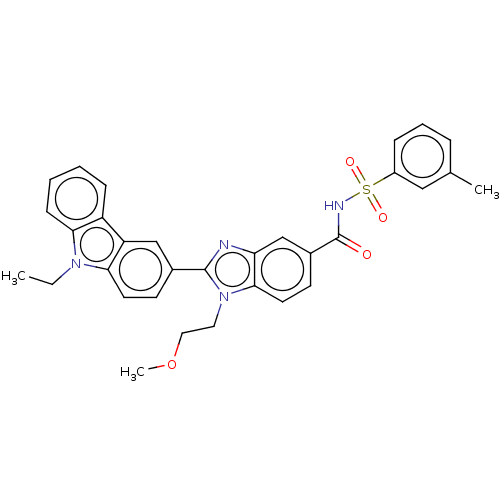 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25.6 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261781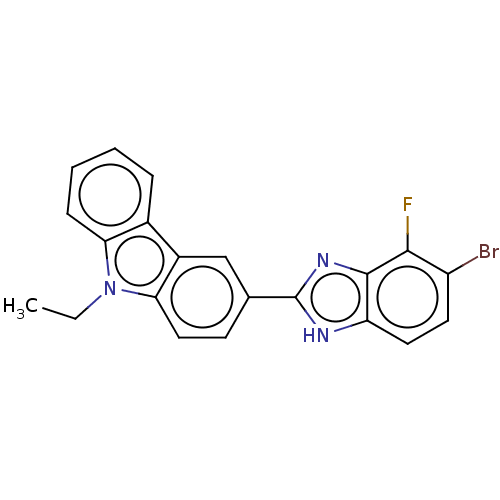 (3-(5-Bromo-4-fluoro-1H-benzimidazol-2-yl)-9-ethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27.4 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261765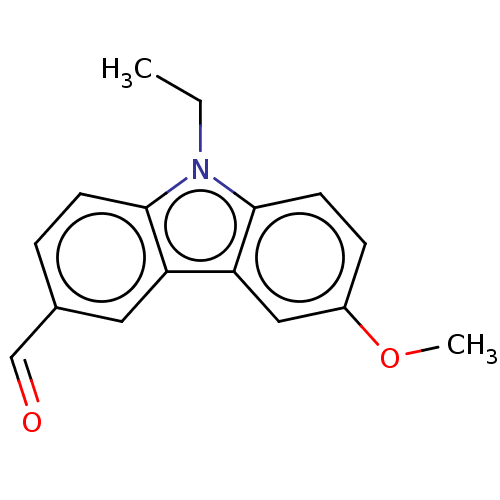 (9-Ethyl-6-methoxy-9H-carbazole-3-carbaldehyde | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28.6 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261782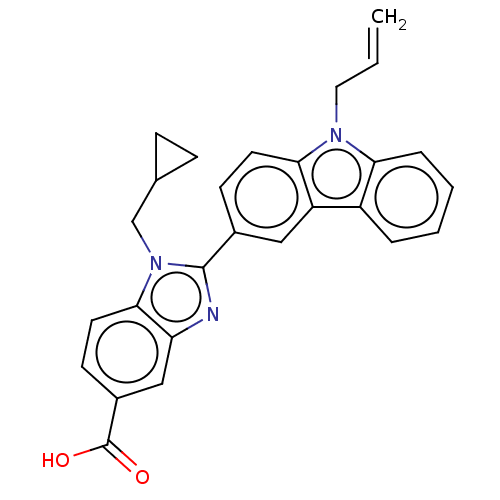 (2-(9-Allyl-9H-carbazol-3-yl)-1-(cyclopropylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28.7 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261779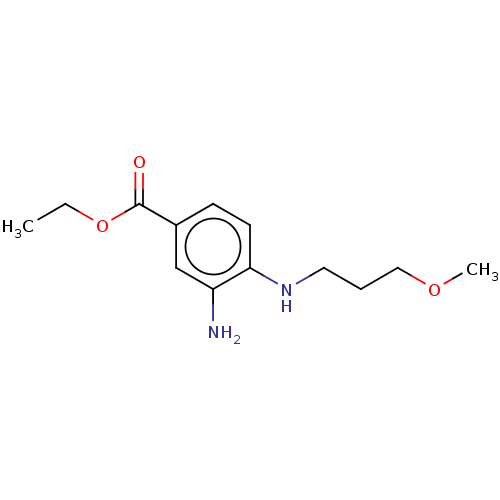 (Ethyl 3-amino-4-[(3-methoxypropyl)amino]benzoate |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 30.1 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261768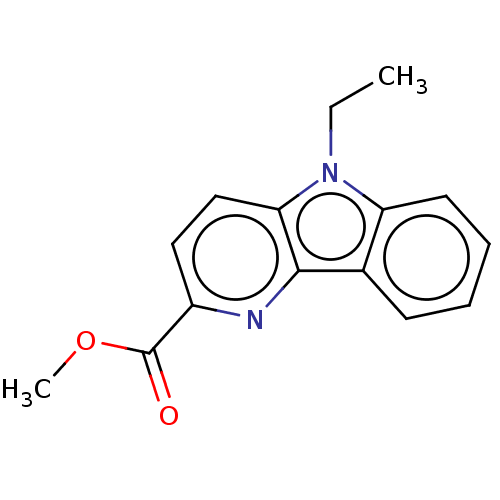 (Methyl 5-ethyl-5H-pyrido[3,2-b]indole-2-carboxylat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32.2 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261763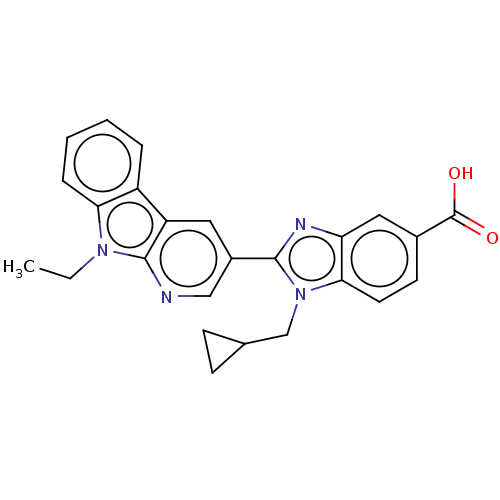 (1-(Cyclopropylmethyl)-2-(9-ethyl-9H-pyrido[2,3-b]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33.4 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261799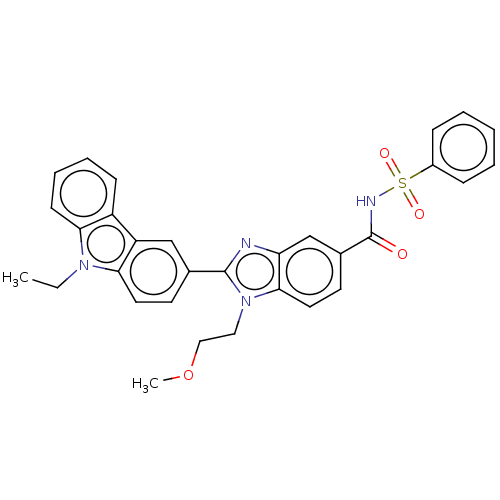 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33.6 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261784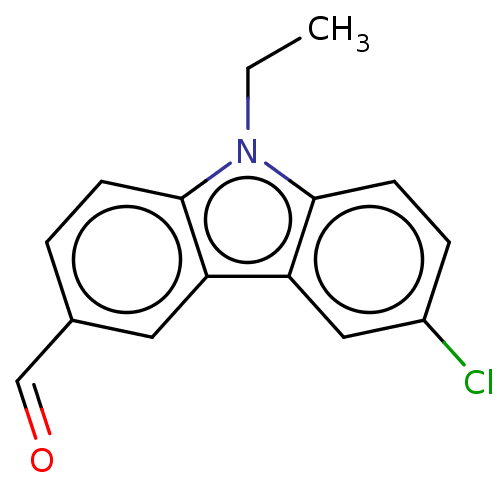 (6-Chloro-9-ethyl-9H-carbazole-3-carbaldehyde and 8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33.8 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261862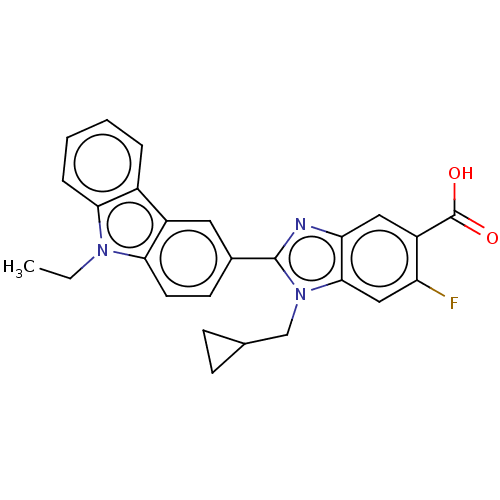 (1-(Cyclopropylmethyl)-2-(9-ethyl-9H-carbazol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34.6 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261802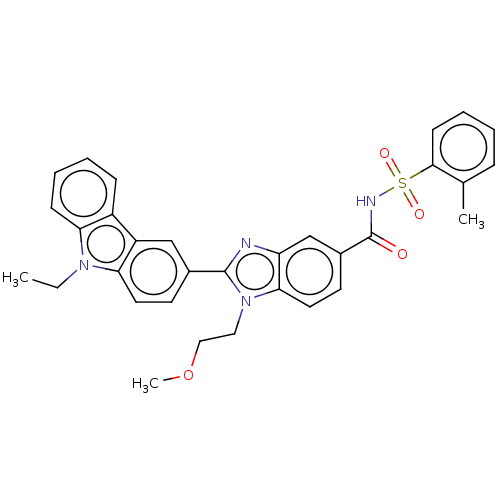 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35.3 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261801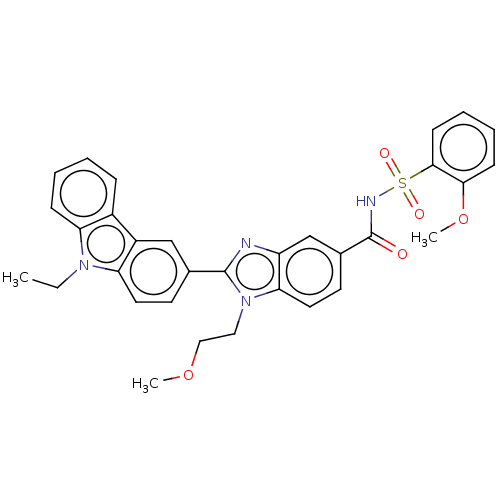 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35.9 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261868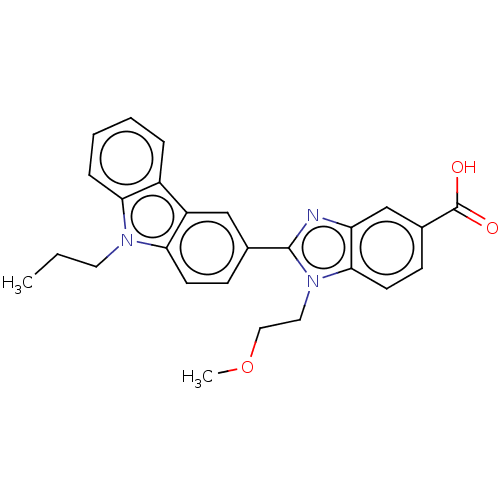 (1-(2-Methoxyethyl)-2-(9-propyl-9H-carbazol-3-yl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.2 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261817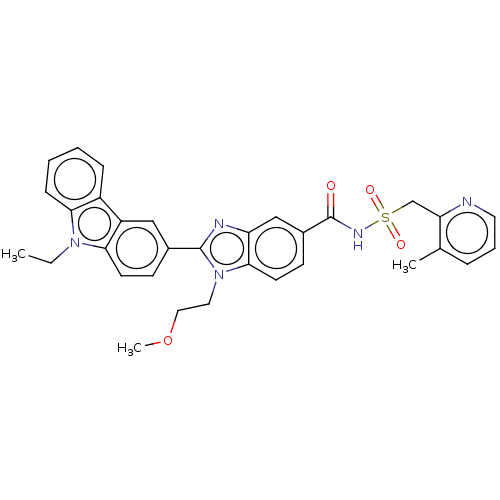 (2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.3 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261816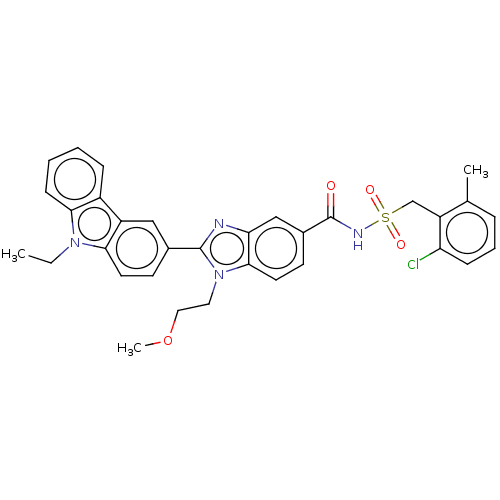 (N-[(2-Chloro-6-methylbenzyl)sulphonyl]-2-(9-ethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.9 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261783 (1-(Cyclopropylmethyl)-2-(9-methyl-9H-carbazol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39.4 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261825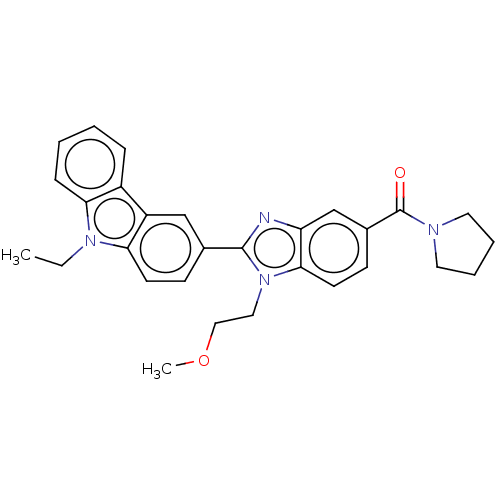 (US9708311, 76 | [2-(9-Ethyl-9H-carbazol-3-yl)-1-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 41.3 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261780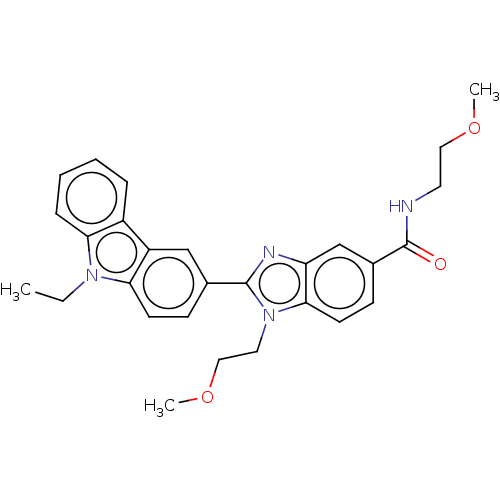 (2-(9-Ethyl-9H-carbazol-3-yl)-N,1-bis(2-methoxyethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42.8 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261869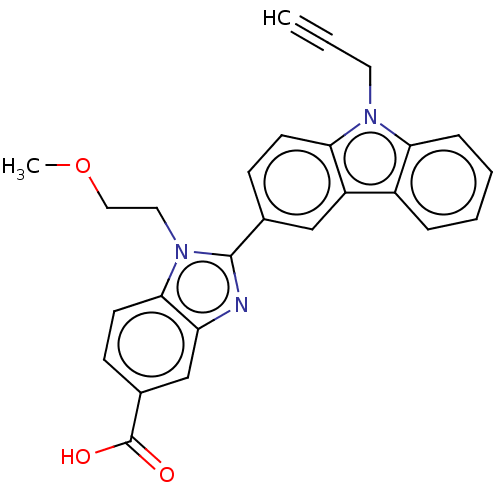 (1-(2-Methoxyethyl)-2-[9-(prop-2-yn-1-yl)-9H-carbaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43.7 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM261856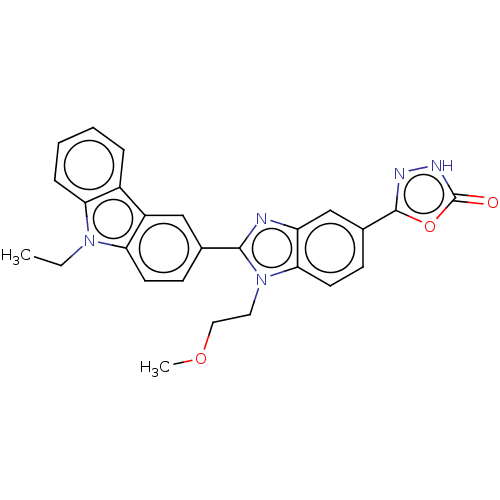 (5-[2-(9-Ethyl-9H-carbazol-3-yl)-1-(2-methoxyethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43.7 | n/a | n/a | n/a | n/a | n/a | 25 |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description 4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (... | US Patent US9708311 (2017) BindingDB Entry DOI: 10.7270/Q23F4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 143 total ) | Next | Last >> |