

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268330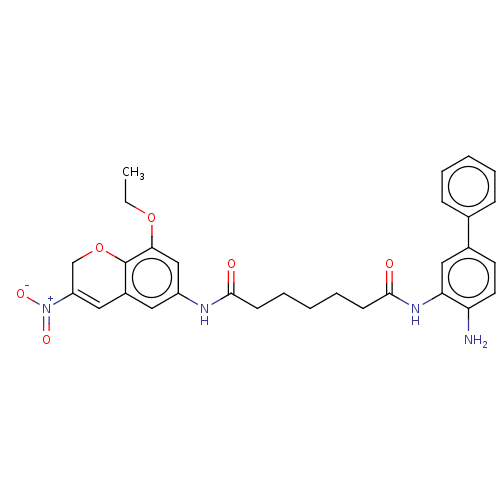 (CHEMBL4060953) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268329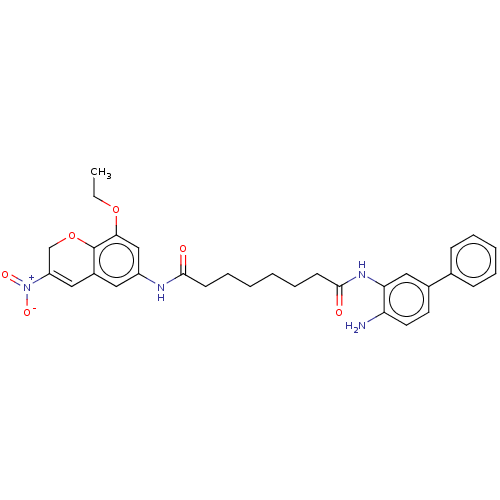 (CHEMBL4095143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268331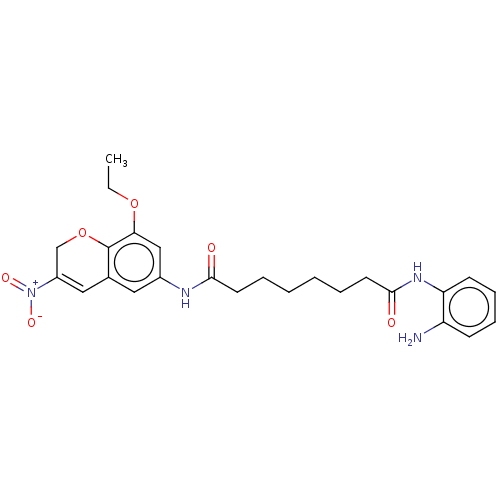 (CHEMBL4069623) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268332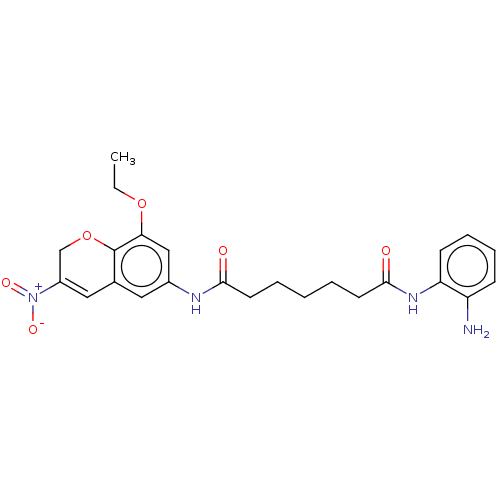 (CHEMBL4103874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268328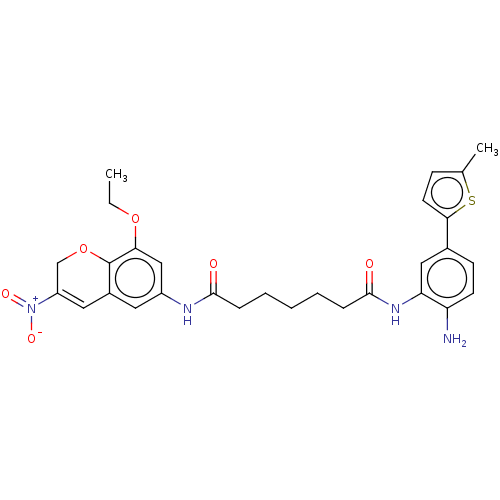 (CHEMBL4072341) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 366 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268331 (CHEMBL4069623) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268327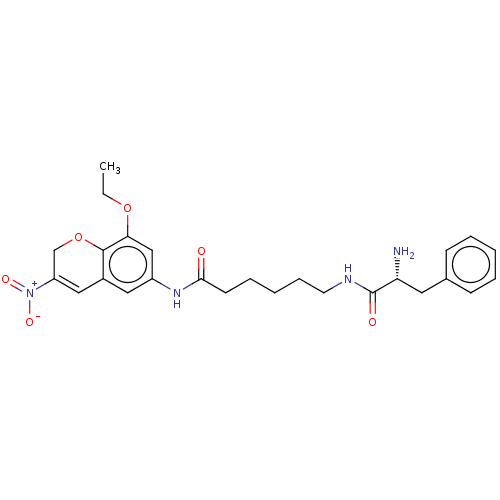 (CHEMBL4075124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268330 (CHEMBL4060953) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 659 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50268319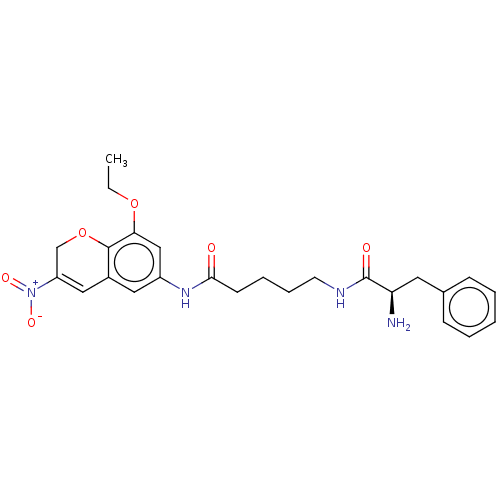 (CHEMBL4103290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 742 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268332 (CHEMBL4103874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268329 (CHEMBL4095143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 827 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268327 (CHEMBL4075124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268328 (CHEMBL4072341) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50268319 (CHEMBL4103290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using Ac-Leu-GlyLys(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition measured after ... | Bioorg Med Chem 25: 4123-4132 (2017) Article DOI: 10.1016/j.bmc.2017.05.062 BindingDB Entry DOI: 10.7270/Q27H1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||