

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273535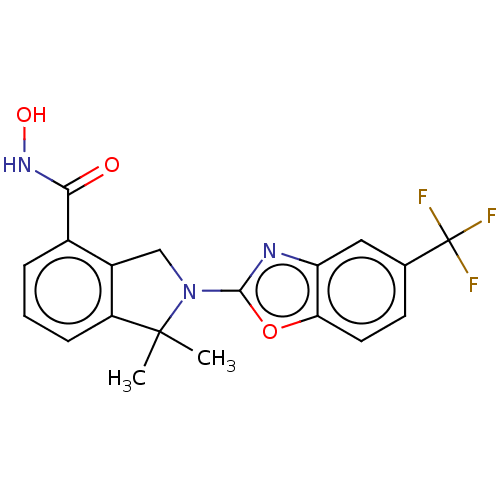 (CHEMBL4130288 | US11535607, Example 18-1) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273525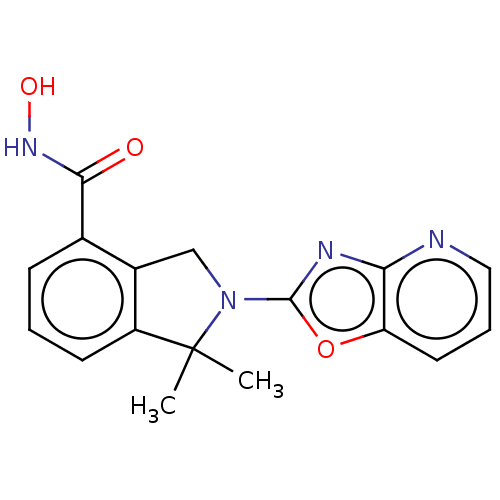 (CHEMBL4128972 | US11535607, Example 18-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50273537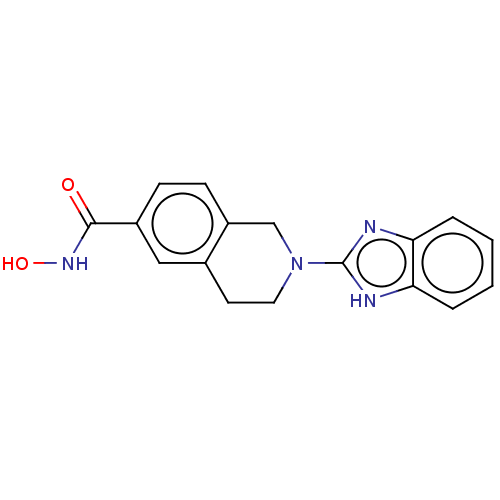 (CHEMBL4128164 | US11535607, Example 50-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273522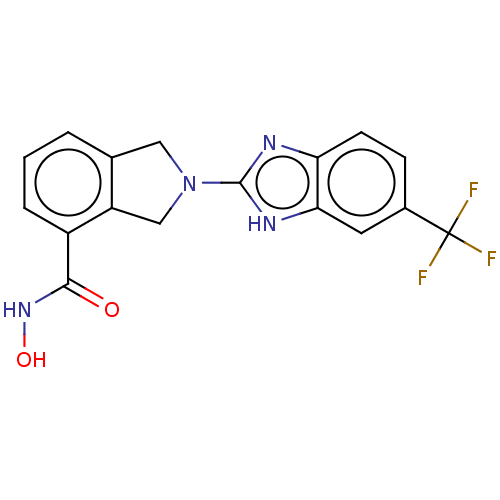 (CHEMBL4127743 | US11535607, Example 12-1) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273553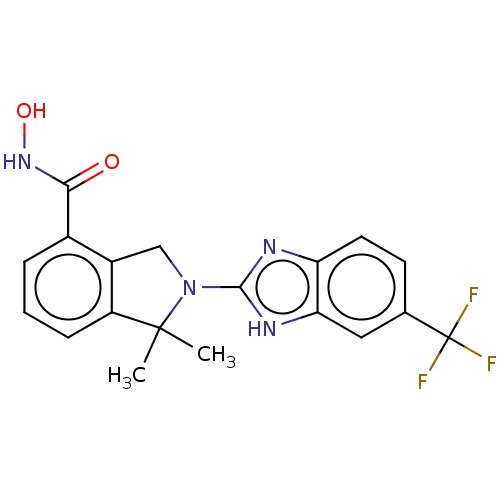 (CHEMBL4127020 | US10508088, ID HDTK028 | US1153560...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273520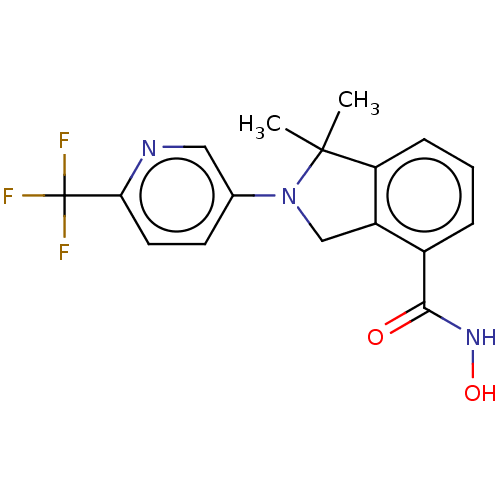 (CHEMBL4127735 | US11535607, Example 22-3) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273526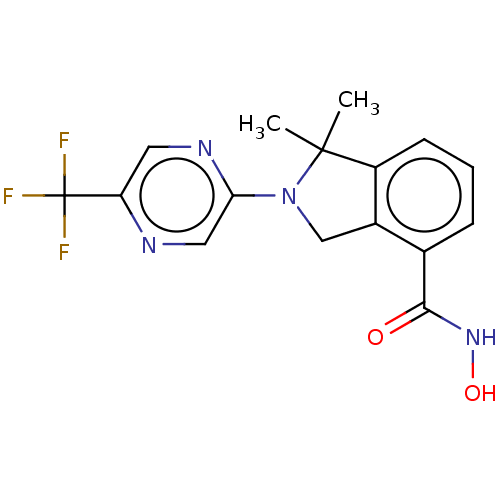 (CHEMBL4126661 | US11535607, Example 22-8) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50273524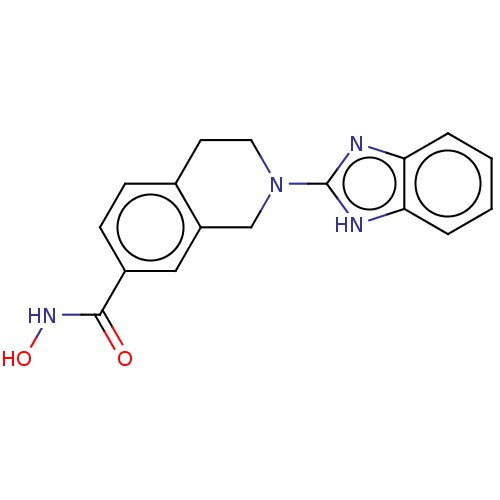 (CHEMBL4129402 | US11535607, Example 50-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273538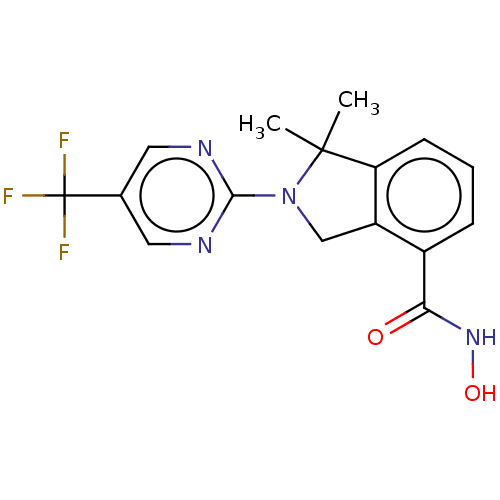 (CHEMBL4127894 | US11535607, Example 22-6) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273554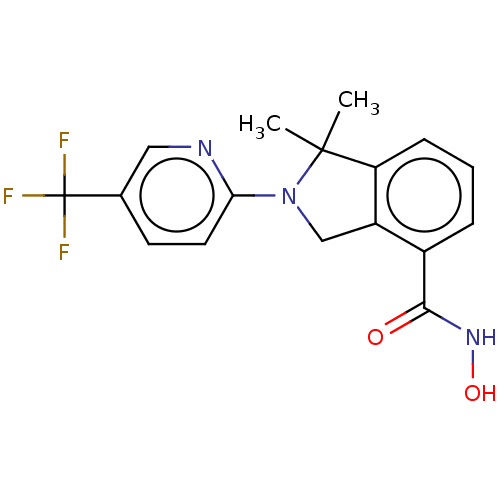 (CHEMBL4129134 | US11535607, Example 22-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273549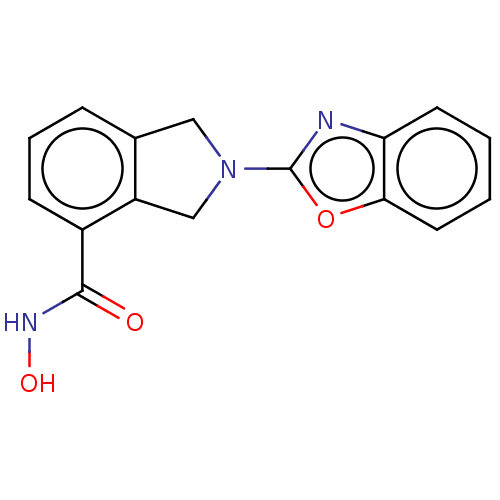 (CHEMBL4129266 | US11535607, Example 15-1) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273551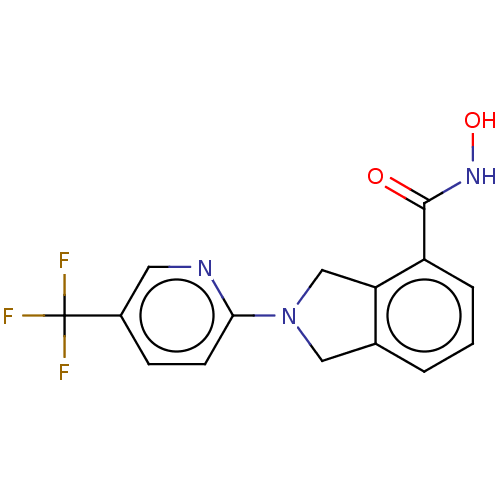 (CHEMBL4129143 | US11535607, Example 45-1) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273520 (CHEMBL4127735 | US11535607, Example 22-3) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273525 (CHEMBL4128972 | US11535607, Example 18-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273523 (CHEMBL4129231 | US11535607, Example 7-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273536 (CHEMBL4126502 | US11535607, Example 22-7) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273521 (CHEMBL4129070 | US11535607, Example 50-3) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273550 (CHEMBL4125954 | US11535607, Example 7-4) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50273539 (CHEMBL4125815 | US11535607, Example 50-6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273538 (CHEMBL4127894 | US11535607, Example 22-6) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273554 (CHEMBL4129134 | US11535607, Example 22-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273540 (CHEMBL4127193 | US11535607, Example 1-6) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273536 (CHEMBL4126502 | US11535607, Example 22-7) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of NanoLuc-tagged HDAC11 (unknown origin) by BRET assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273552 (CHEMBL4125788) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273533 (CHEMBL4126784 | US11535607, Example 50-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273524 (CHEMBL4129402 | US11535607, Example 50-4) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273534 (CHEMBL4127829 | US11535607, Example 1-2) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50273521 (CHEMBL4129070 | US11535607, Example 50-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273539 (CHEMBL4125815 | US11535607, Example 50-6) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC8 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50273540 (CHEMBL4127193 | US11535607, Example 1-6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50273533 (CHEMBL4126784 | US11535607, Example 50-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC6 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273519 (CHEMBL4129930) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50273537 (CHEMBL4128164 | US11535607, Example 50-5) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC11 expressed in baculoviral expression system using FAM-RHKK as substrate by electrophoretic mobility... | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC3 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC10 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50273519 (CHEMBL4129930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC8 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50273519 (CHEMBL4129930) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC5 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50273519 (CHEMBL4129930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC2 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC4 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50273519 (CHEMBL4129930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC1 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50273519 (CHEMBL4129930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC7 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC1 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC2 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50273519 (CHEMBL4129930) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC3 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50273519 (CHEMBL4129930) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC4 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC5 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50273526 (CHEMBL4126661 | US11535607, Example 22-8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC7 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50273519 (CHEMBL4129930) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
FORMA Therapeutics Curated by ChEMBL | Assay Description Inhibition of full length recombinant human HDAC9 using fluorescent-labeled peptide as substrate by electrophoretic mobility shift assay | Bioorg Med Chem Lett 28: 2143-2147 (2018) Article DOI: 10.1016/j.bmcl.2018.05.021 BindingDB Entry DOI: 10.7270/Q2PR7ZGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |