 Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50001897
Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50001897 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449913
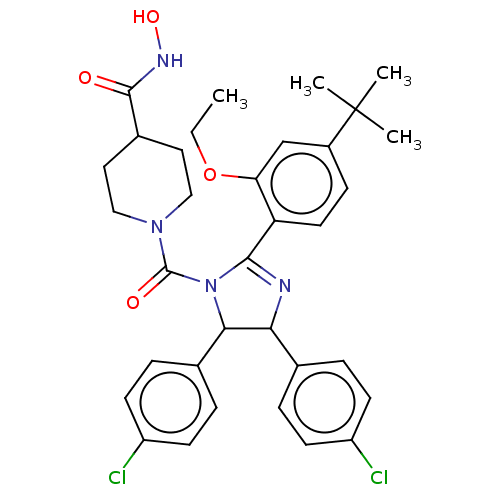
(CHEMBL4170056)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCC(CC1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C34H38Cl2N4O4/c1-5-44-28-20-24(34(2,3)4)10-15-27(28)31-37-29(21-6-11-25(35)12-7-21)30(22-8-13-26(36)14-9-22)40(31)33(42)39-18-16-23(17-19-39)32(41)38-43/h6-15,20,23,29-30,43H,5,16-19H2,1-4H3,(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449907
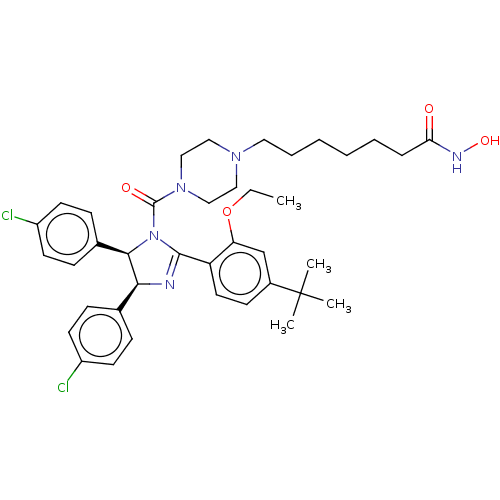
(CHEMBL4163675)Show SMILES CCOc1cc(ccc1C1=N[C@H]([C@H](N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47)/t35-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449901
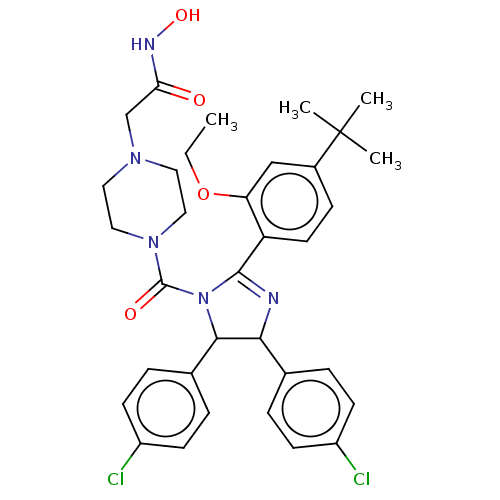
(CHEMBL4160754)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C34H39Cl2N5O4/c1-5-45-28-20-24(34(2,3)4)10-15-27(28)32-37-30(22-6-11-25(35)12-7-22)31(23-8-13-26(36)14-9-23)41(32)33(43)40-18-16-39(17-19-40)21-29(42)38-44/h6-15,20,30-31,44H,5,16-19,21H2,1-4H3,(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449900
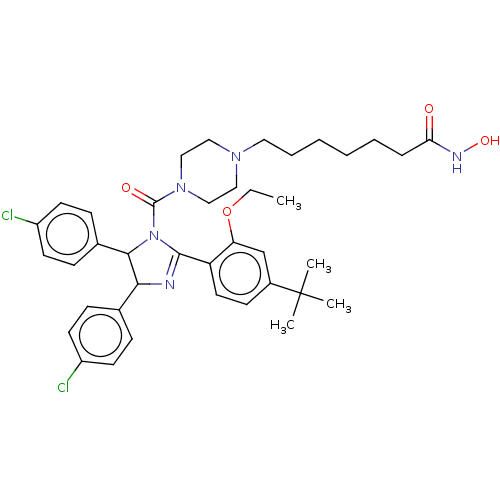
(CHEMBL4174805)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449896
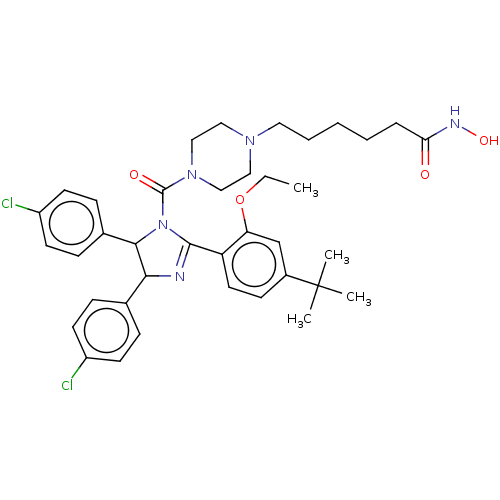
(CHEMBL4164148)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C38H47Cl2N5O4/c1-5-49-32-25-28(38(2,3)4)14-19-31(32)36-41-34(26-10-15-29(39)16-11-26)35(27-12-17-30(40)18-13-27)45(36)37(47)44-23-21-43(22-24-44)20-8-6-7-9-33(46)42-48/h10-19,25,34-35,48H,5-9,20-24H2,1-4H3,(H,42,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449924
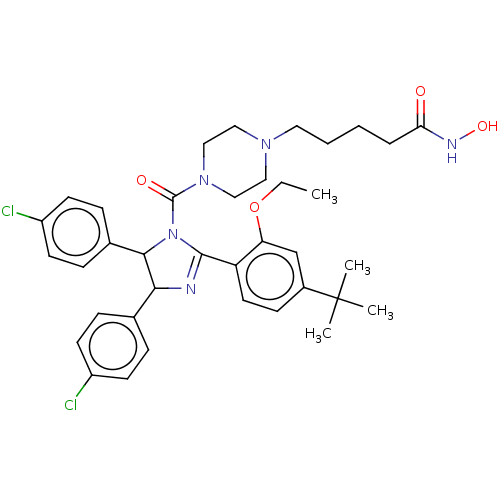
(CHEMBL4171353)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C37H45Cl2N5O4/c1-5-48-31-24-27(37(2,3)4)13-18-30(31)35-40-33(25-9-14-28(38)15-10-25)34(26-11-16-29(39)17-12-26)44(35)36(46)43-22-20-42(21-23-43)19-7-6-8-32(45)41-47/h9-18,24,33-34,47H,5-8,19-23H2,1-4H3,(H,41,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449923
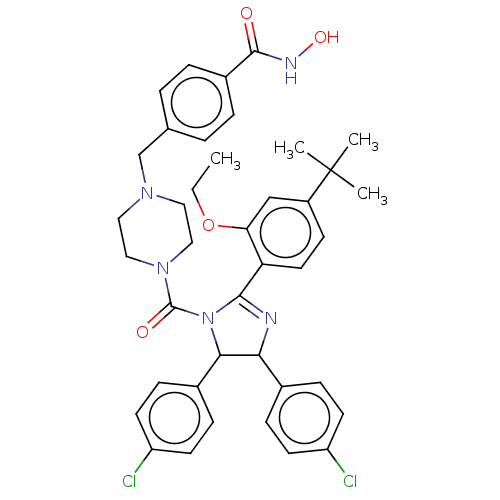
(CHEMBL4163017)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(Cc2ccc(cc2)C(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C40H43Cl2N5O4/c1-5-51-34-24-30(40(2,3)4)14-19-33(34)37-43-35(27-10-15-31(41)16-11-27)36(28-12-17-32(42)18-13-28)47(37)39(49)46-22-20-45(21-23-46)25-26-6-8-29(9-7-26)38(48)44-50/h6-19,24,35-36,50H,5,20-23,25H2,1-4H3,(H,44,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449903
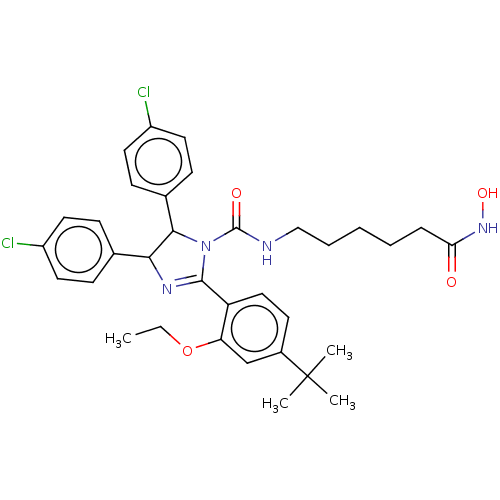
(CHEMBL4170573)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C34H40Cl2N4O4/c1-5-44-28-21-24(34(2,3)4)14-19-27(28)32-38-30(22-10-15-25(35)16-11-22)31(23-12-17-26(36)18-13-23)40(32)33(42)37-20-8-6-7-9-29(41)39-43/h10-19,21,30-31,43H,5-9,20H2,1-4H3,(H,37,42)(H,39,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449895
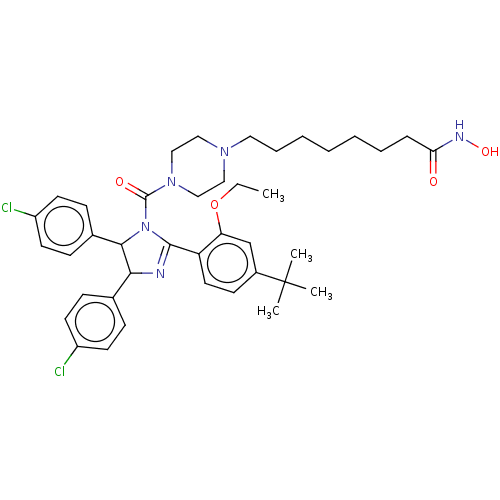
(CHEMBL4173404)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C40H51Cl2N5O4/c1-5-51-34-27-30(40(2,3)4)16-21-33(34)38-43-36(28-12-17-31(41)18-13-28)37(29-14-19-32(42)20-15-29)47(38)39(49)46-25-23-45(24-26-46)22-10-8-6-7-9-11-35(48)44-50/h12-21,27,36-37,50H,5-11,22-26H2,1-4H3,(H,44,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449925
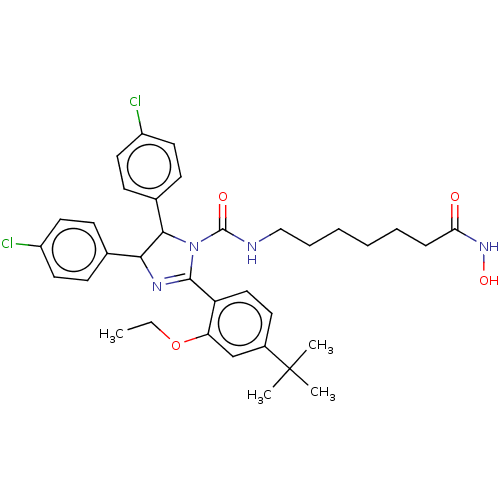
(CHEMBL4159921)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C35H42Cl2N4O4/c1-5-45-29-22-25(35(2,3)4)15-20-28(29)33-39-31(23-11-16-26(36)17-12-23)32(24-13-18-27(37)19-14-24)41(33)34(43)38-21-9-7-6-8-10-30(42)40-44/h11-20,22,31-32,44H,5-10,21H2,1-4H3,(H,38,43)(H,40,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449898
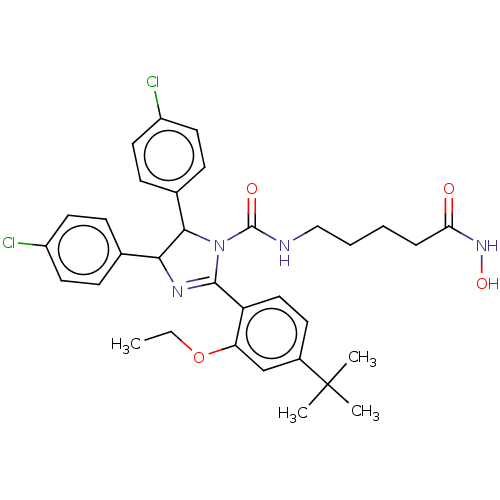
(CHEMBL4166471)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C33H38Cl2N4O4/c1-5-43-27-20-23(33(2,3)4)13-18-26(27)31-37-29(21-9-14-24(34)15-10-21)30(22-11-16-25(35)17-12-22)39(31)32(41)36-19-7-6-8-28(40)38-42/h9-18,20,29-30,42H,5-8,19H2,1-4H3,(H,36,41)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449897
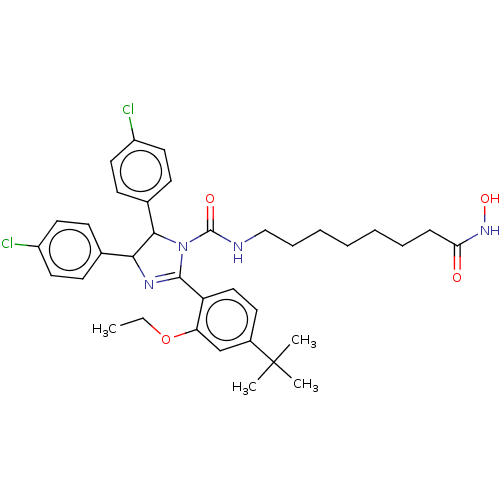
(CHEMBL4163120)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C36H44Cl2N4O4/c1-5-46-30-23-26(36(2,3)4)16-21-29(30)34-40-32(24-12-17-27(37)18-13-24)33(25-14-19-28(38)20-15-25)42(34)35(44)39-22-10-8-6-7-9-11-31(43)41-45/h12-21,23,32-33,45H,5-11,22H2,1-4H3,(H,39,44)(H,41,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449894
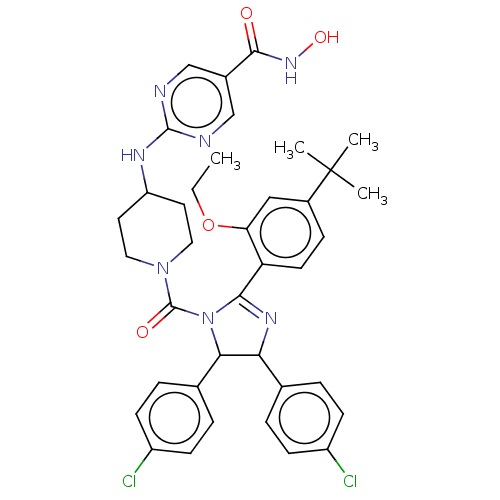
(CHEMBL4163059)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCC(CC1)Nc1ncc(cn1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C38H41Cl2N7O4/c1-5-51-31-20-26(38(2,3)4)10-15-30(31)34-44-32(23-6-11-27(39)12-7-23)33(24-8-13-28(40)14-9-24)47(34)37(49)46-18-16-29(17-19-46)43-36-41-21-25(22-42-36)35(48)45-50/h6-15,20-22,29,32-33,50H,5,16-19H2,1-4H3,(H,45,48)(H,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449902
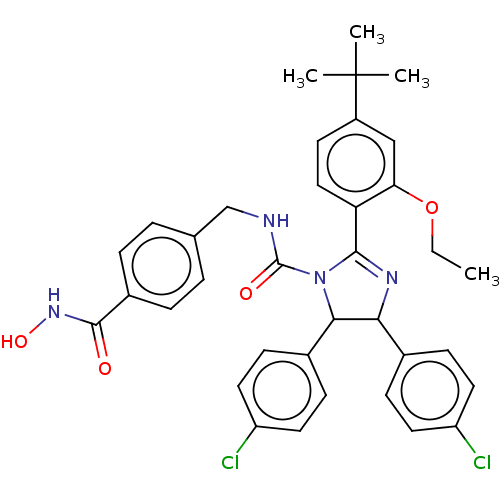
(CHEMBL4173507)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCc1ccc(cc1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C36H36Cl2N4O4/c1-5-46-30-20-26(36(2,3)4)14-19-29(30)33-40-31(23-10-15-27(37)16-11-23)32(24-12-17-28(38)18-13-24)42(33)35(44)39-21-22-6-8-25(9-7-22)34(43)41-45/h6-20,31-32,45H,5,21H2,1-4H3,(H,39,44)(H,41,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449905
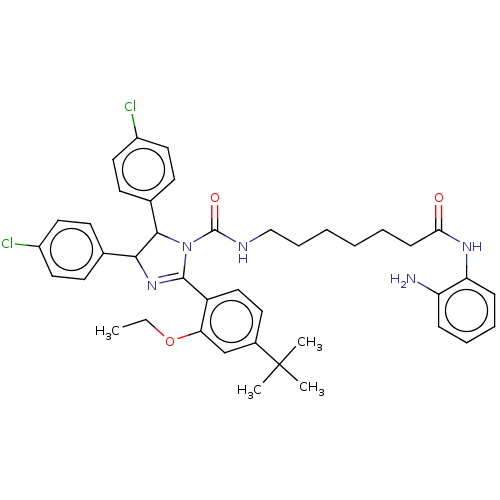
(CHEMBL4171605)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCC(=O)Nc1ccccc1N)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C41H47Cl2N5O3/c1-5-51-35-26-29(41(2,3)4)19-24-32(35)39-47-37(27-15-20-30(42)21-16-27)38(28-17-22-31(43)23-18-28)48(39)40(50)45-25-11-7-6-8-14-36(49)46-34-13-10-9-12-33(34)44/h9-10,12-13,15-24,26,37-38H,5-8,11,14,25,44H2,1-4H3,(H,45,50)(H,46,49) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449899
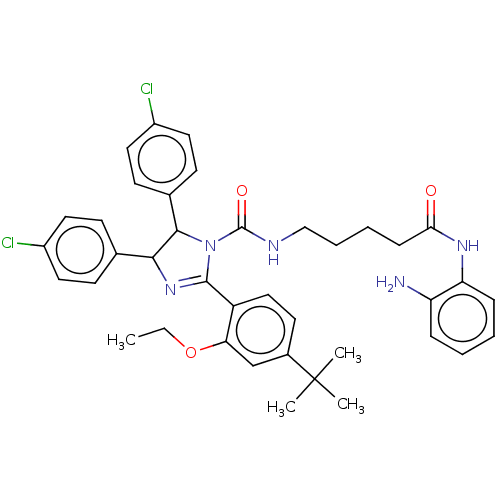
(CHEMBL4175049)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCC(=O)Nc1ccccc1N)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H43Cl2N5O3/c1-5-49-33-24-27(39(2,3)4)17-22-30(33)37-45-35(25-13-18-28(40)19-14-25)36(26-15-20-29(41)21-16-26)46(37)38(48)43-23-9-8-12-34(47)44-32-11-7-6-10-31(32)42/h6-7,10-11,13-22,24,35-36H,5,8-9,12,23,42H2,1-4H3,(H,43,48)(H,44,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449904
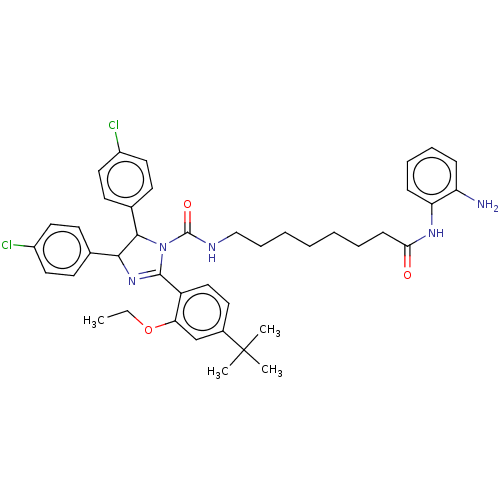
(CHEMBL4174687)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCCC(=O)Nc1ccccc1N)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C42H49Cl2N5O3/c1-5-52-36-27-30(42(2,3)4)20-25-33(36)40-48-38(28-16-21-31(43)22-17-28)39(29-18-23-32(44)24-19-29)49(40)41(51)46-26-12-8-6-7-9-15-37(50)47-35-14-11-10-13-34(35)45/h10-11,13-14,16-25,27,38-39H,5-9,12,15,26,45H2,1-4H3,(H,46,51)(H,47,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449922
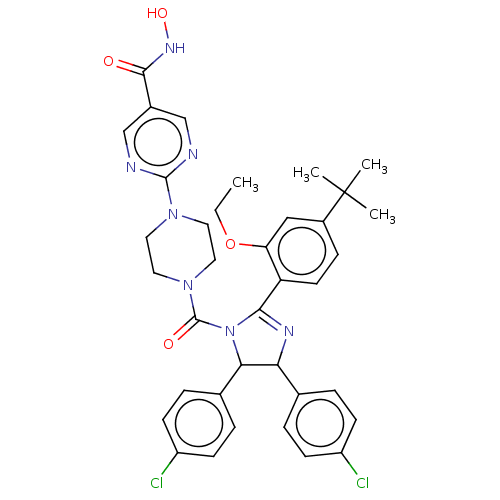
(CHEMBL4170975)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CC1)c1ncc(cn1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C37H39Cl2N7O4/c1-5-50-30-20-26(37(2,3)4)10-15-29(30)33-42-31(23-6-11-27(38)12-7-23)32(24-8-13-28(39)14-9-24)46(33)36(48)45-18-16-44(17-19-45)35-40-21-25(22-41-35)34(47)43-49/h6-15,20-22,31-32,49H,5,16-19H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449906
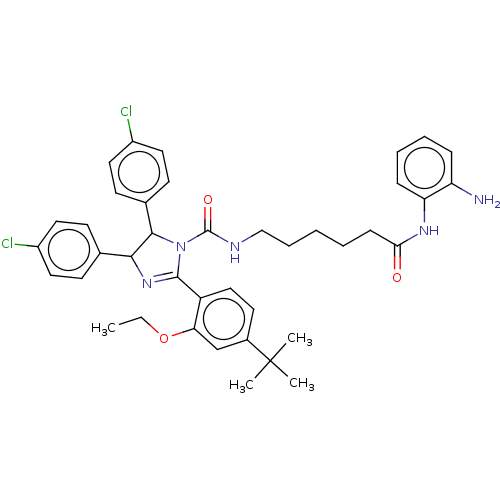
(CHEMBL4163726)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCC(=O)Nc1ccccc1N)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C40H45Cl2N5O3/c1-5-50-34-25-28(40(2,3)4)18-23-31(34)38-46-36(26-14-19-29(41)20-15-26)37(27-16-21-30(42)22-17-27)47(38)39(49)44-24-10-6-7-13-35(48)45-33-12-9-8-11-32(33)43/h8-9,11-12,14-23,25,36-37H,5-7,10,13,24,43H2,1-4H3,(H,44,49)(H,45,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449921
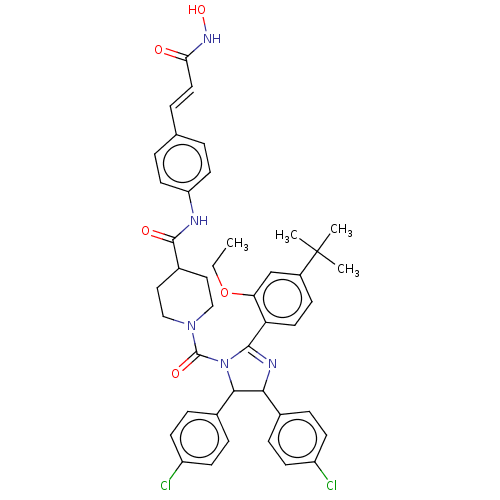
(CHEMBL4161826)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCC(CC1)C(=O)Nc1ccc(\C=C\C(=O)NO)cc1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C43H45Cl2N5O5/c1-5-55-36-26-31(43(2,3)4)13-20-35(36)40-47-38(28-9-14-32(44)15-10-28)39(29-11-16-33(45)17-12-29)50(40)42(53)49-24-22-30(23-25-49)41(52)46-34-18-6-27(7-19-34)8-21-37(51)48-54/h6-21,26,30,38-39,54H,5,22-25H2,1-4H3,(H,46,52)(H,48,51)/b21-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449908
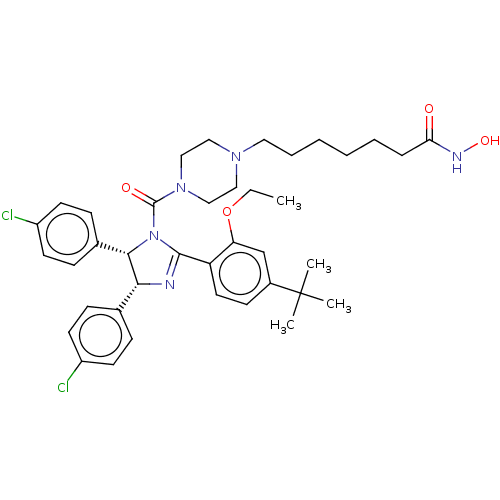
(CHEMBL4168217)Show SMILES CCOc1cc(ccc1C1=N[C@@H]([C@@H](N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47)/t35-,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50449900

(CHEMBL4174805)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50449900

(CHEMBL4174805)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449897

(CHEMBL4163120)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C36H44Cl2N4O4/c1-5-46-30-23-26(36(2,3)4)16-21-29(30)34-40-32(24-12-17-27(37)18-13-24)33(25-14-19-28(38)20-15-25)42(34)35(44)39-22-10-8-6-7-9-11-31(43)41-45/h12-21,23,32-33,45H,5-11,22H2,1-4H3,(H,39,44)(H,41,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449922

(CHEMBL4170975)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CC1)c1ncc(cn1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C37H39Cl2N7O4/c1-5-50-30-20-26(37(2,3)4)10-15-29(30)33-42-31(23-6-11-27(38)12-7-23)32(24-8-13-28(39)14-9-24)46(33)36(48)45-18-16-44(17-19-45)35-40-21-25(22-41-35)34(47)43-49/h6-15,20-22,31-32,49H,5,16-19H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449895

(CHEMBL4173404)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C40H51Cl2N5O4/c1-5-51-34-27-30(40(2,3)4)16-21-33(34)38-43-36(28-12-17-31(41)18-13-28)37(29-14-19-32(42)20-15-29)47(38)39(49)46-25-23-45(24-26-46)22-10-8-6-7-9-11-35(48)44-50/h12-21,27,36-37,50H,5-11,22-26H2,1-4H3,(H,44,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50449900

(CHEMBL4174805)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 421 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449900

(CHEMBL4174805)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449907

(CHEMBL4163675)Show SMILES CCOc1cc(ccc1C1=N[C@H]([C@H](N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47)/t35-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449925

(CHEMBL4159921)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C35H42Cl2N4O4/c1-5-45-29-22-25(35(2,3)4)15-20-28(29)33-39-31(23-11-16-26(36)17-12-23)32(24-13-18-27(37)19-14-24)41(33)34(43)38-21-9-7-6-8-10-30(42)40-44/h11-20,22,31-32,44H,5-10,21H2,1-4H3,(H,38,43)(H,40,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449908

(CHEMBL4168217)Show SMILES CCOc1cc(ccc1C1=N[C@@H]([C@@H](N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47)/t35-,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449921

(CHEMBL4161826)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCC(CC1)C(=O)Nc1ccc(\C=C\C(=O)NO)cc1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C43H45Cl2N5O5/c1-5-55-36-26-31(43(2,3)4)13-20-35(36)40-47-38(28-9-14-32(44)15-10-28)39(29-11-16-33(45)17-12-29)50(40)42(53)49-24-22-30(23-25-49)41(52)46-34-18-6-27(7-19-34)8-21-37(51)48-54/h6-21,26,30,38-39,54H,5,22-25H2,1-4H3,(H,46,52)(H,48,51)/b21-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449896

(CHEMBL4164148)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C38H47Cl2N5O4/c1-5-49-32-25-28(38(2,3)4)14-19-31(32)36-41-34(26-10-15-29(39)16-11-26)35(27-12-17-30(40)18-13-27)45(36)37(47)44-23-21-43(22-24-44)20-8-6-7-9-33(46)42-48/h10-19,25,34-35,48H,5-9,20-24H2,1-4H3,(H,42,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449924

(CHEMBL4171353)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C37H45Cl2N5O4/c1-5-48-31-24-27(37(2,3)4)13-18-30(31)35-40-33(25-9-14-28(38)15-10-25)34(26-11-16-29(39)17-12-26)44(35)36(46)43-22-20-42(21-23-43)19-7-6-8-32(45)41-47/h9-18,24,33-34,47H,5-8,19-23H2,1-4H3,(H,41,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449894

(CHEMBL4163059)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCC(CC1)Nc1ncc(cn1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C38H41Cl2N7O4/c1-5-51-31-20-26(38(2,3)4)10-15-30(31)34-44-32(23-6-11-27(39)12-7-23)33(24-8-13-28(40)14-9-24)47(34)37(49)46-18-16-29(17-19-46)43-36-41-21-25(22-42-36)35(48)45-50/h6-15,20-22,29,32-33,50H,5,16-19H2,1-4H3,(H,45,48)(H,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449903

(CHEMBL4170573)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C34H40Cl2N4O4/c1-5-44-28-21-24(34(2,3)4)14-19-27(28)32-38-30(22-10-15-25(35)16-11-22)31(23-12-17-26(36)18-13-23)40(32)33(42)37-20-8-6-7-9-29(41)39-43/h10-19,21,30-31,43H,5-9,20H2,1-4H3,(H,37,42)(H,39,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50449900

(CHEMBL4174805)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449898

(CHEMBL4166471)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C33H38Cl2N4O4/c1-5-43-27-20-23(33(2,3)4)13-18-26(27)31-37-29(21-9-14-24(34)15-10-21)30(22-11-16-25(35)17-12-22)39(31)32(41)36-19-7-6-8-28(40)38-42/h9-18,20,29-30,42H,5-8,19H2,1-4H3,(H,36,41)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449923

(CHEMBL4163017)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(Cc2ccc(cc2)C(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C40H43Cl2N5O4/c1-5-51-34-24-30(40(2,3)4)14-19-33(34)37-43-35(27-10-15-31(41)16-11-27)36(28-12-17-32(42)18-13-28)47(37)39(49)46-22-20-45(21-23-46)25-26-6-8-29(9-7-26)38(48)44-50/h6-19,24,35-36,50H,5,20-23,25H2,1-4H3,(H,44,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449904

(CHEMBL4174687)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCCC(=O)Nc1ccccc1N)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C42H49Cl2N5O3/c1-5-52-36-27-30(42(2,3)4)20-25-33(36)40-48-38(28-16-21-31(43)22-17-28)39(29-18-23-32(44)24-19-29)49(40)41(51)46-26-12-8-6-7-9-15-37(50)47-35-14-11-10-13-34(35)45/h10-11,13-14,16-25,27,38-39H,5-9,12,15,26,45H2,1-4H3,(H,46,51)(H,47,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449902

(CHEMBL4173507)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCc1ccc(cc1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C36H36Cl2N4O4/c1-5-46-30-20-26(36(2,3)4)14-19-29(30)33-40-31(23-10-15-27(37)16-11-23)32(24-12-17-28(38)18-13-24)42(33)35(44)39-21-22-6-8-25(9-7-22)34(43)41-45/h6-20,31-32,45H,5,21H2,1-4H3,(H,39,44)(H,41,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449901

(CHEMBL4160754)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C34H39Cl2N5O4/c1-5-45-28-20-24(34(2,3)4)10-15-27(28)32-37-30(22-6-11-25(35)12-7-22)31(23-8-13-26(36)14-9-23)41(32)33(43)40-18-16-39(17-19-40)21-29(42)38-44/h6-15,20,30-31,44H,5,16-19,21H2,1-4H3,(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data