 Found 21 hits Enz. Inhib. hit(s) with all data for entry = 50003281
Found 21 hits Enz. Inhib. hit(s) with all data for entry = 50003281 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468986
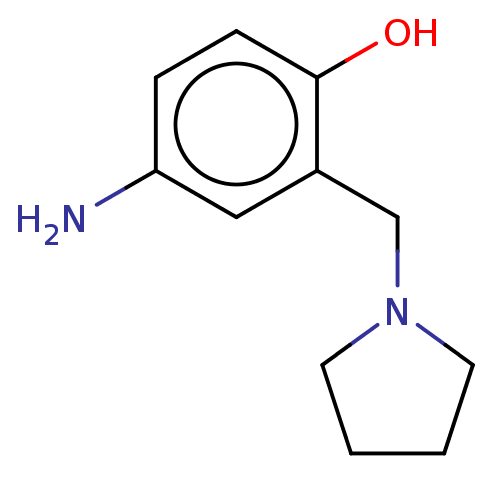
(CHEMBL4282163)Show InChI InChI=1S/C11H16N2O/c12-10-3-4-11(14)9(7-10)8-13-5-1-2-6-13/h3-4,7,14H,1-2,5-6,8,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of AChE in human erythrocytes using varying levels of acetylthiocholine as substrate measured for 1 min by Lineweaver-burk... |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50455616
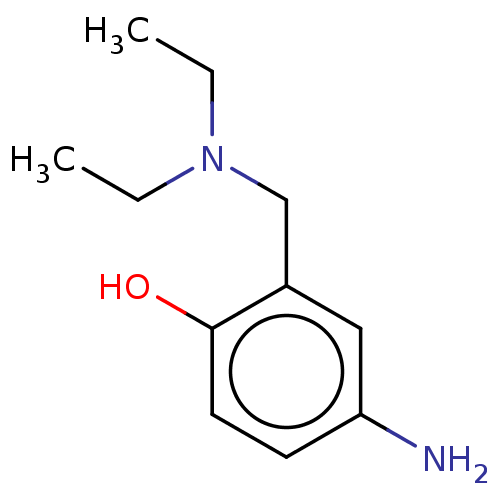
(CHEMBL4215993)Show InChI InChI=1S/C11H16N2O4S2/c1-2-16-4-3-13-7-9-5-8-6-10(19(12,14)15)17-11(8)18-9/h5-6,13H,2-4,7H2,1H3,(H2,12,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human AChE using varying levels of acetylthiocholine as substrate |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468986

(CHEMBL4282163)Show InChI InChI=1S/C11H16N2O/c12-10-3-4-11(14)9(7-10)8-13-5-1-2-6-13/h3-4,7,14H,1-2,5-6,8,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Competitive inhibition of AChE in human erythrocytes using varying levels of acetylthiocholine as substrate measured for 1 min by Lineweaver-burk plo... |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50455616

(CHEMBL4215993)Show InChI InChI=1S/C11H16N2O4S2/c1-2-16-4-3-13-7-9-5-8-6-10(19(12,14)15)17-11(8)18-9/h5-6,13H,2-4,7H2,1H3,(H2,12,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Competitive inhibition of AChE in human erythrocytes using varying levels of acetylthiocholine as substrate measured for 1 min by Lineweaver-burk plo... |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50455616

(CHEMBL4215993)Show InChI InChI=1S/C11H16N2O4S2/c1-2-16-4-3-13-7-9-5-8-6-10(19(12,14)15)17-11(8)18-9/h5-6,13H,2-4,7H2,1H3,(H2,12,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant human AChE using varying levels of acetylthiocholine as substrate |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50455616

(CHEMBL4215993)Show InChI InChI=1S/C11H16N2O4S2/c1-2-16-4-3-13-7-9-5-8-6-10(19(12,14)15)17-11(8)18-9/h5-6,13H,2-4,7H2,1H3,(H2,12,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of AChE in human erythrocytes using varying levels of acetylthiocholine as substrate measured for 1 min by Lineweaver-burk... |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468986

(CHEMBL4282163)Show InChI InChI=1S/C11H16N2O/c12-10-3-4-11(14)9(7-10)8-13-5-1-2-6-13/h3-4,7,14H,1-2,5-6,8,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50455616

(CHEMBL4215993)Show InChI InChI=1S/C11H16N2O4S2/c1-2-16-4-3-13-7-9-5-8-6-10(19(12,14)15)17-11(8)18-9/h5-6,13H,2-4,7H2,1H3,(H2,12,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB reagent based assay |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50455616

(CHEMBL4215993)Show InChI InChI=1S/C11H16N2O4S2/c1-2-16-4-3-13-7-9-5-8-6-10(19(12,14)15)17-11(8)18-9/h5-6,13H,2-4,7H2,1H3,(H2,12,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468994
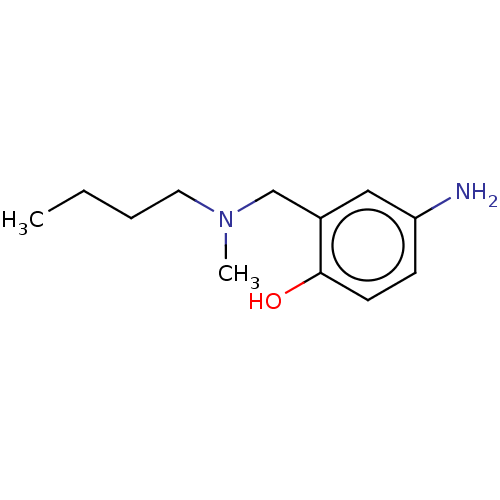
(CHEMBL4280848)Show InChI InChI=1S/C12H20N2O/c1-3-4-7-14(2)9-10-8-11(13)5-6-12(10)15/h5-6,8,15H,3-4,7,9,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468990
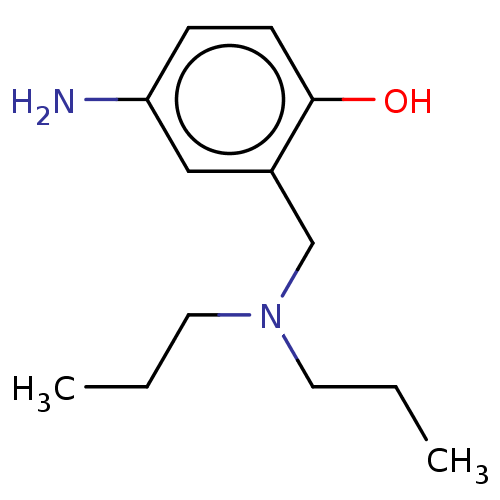
(CHEMBL4285213)Show InChI InChI=1S/C13H22N2O/c1-3-7-15(8-4-2)10-11-9-12(14)5-6-13(11)16/h5-6,9,16H,3-4,7-8,10,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468997
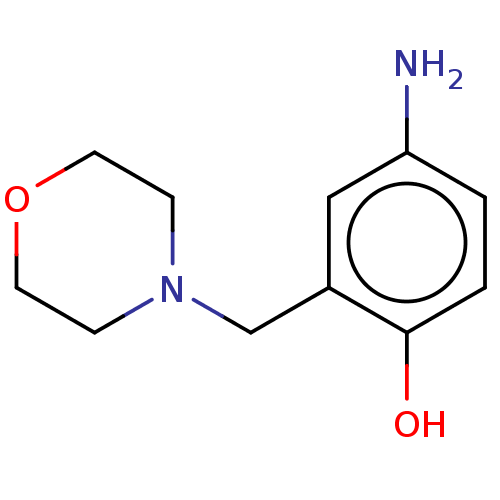
(CHEMBL4294906)Show InChI InChI=1S/C11H16N2O2/c12-10-1-2-11(14)9(7-10)8-13-3-5-15-6-4-13/h1-2,7,14H,3-6,8,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468993
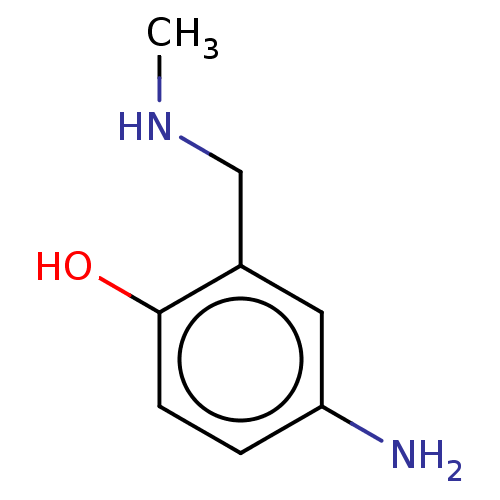
(CHEMBL4292574)Show InChI InChI=1S/C8H12N2O/c1-10-5-6-4-7(9)2-3-8(6)11/h2-4,10-11H,5,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468998
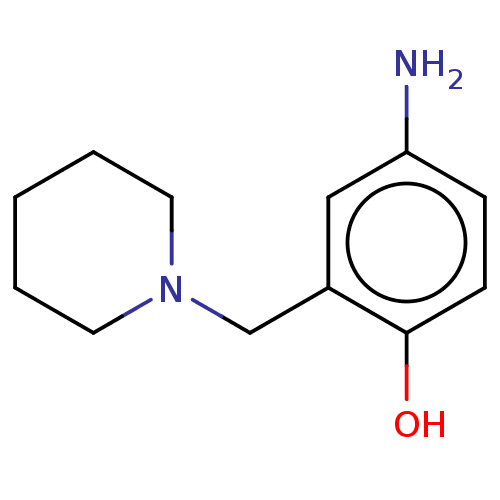
(CHEMBL4288558)Show InChI InChI=1S/C12H18N2O/c13-11-4-5-12(15)10(8-11)9-14-6-2-1-3-7-14/h4-5,8,15H,1-3,6-7,9,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468989
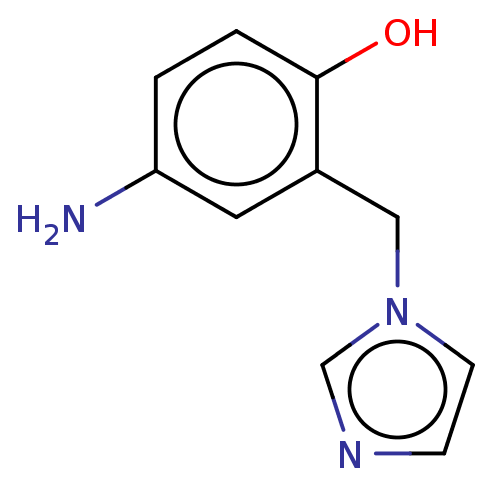
(CHEMBL4286677)Show InChI InChI=1S/C10H11N3O/c11-9-1-2-10(14)8(5-9)6-13-4-3-12-7-13/h1-5,7,14H,6,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468991
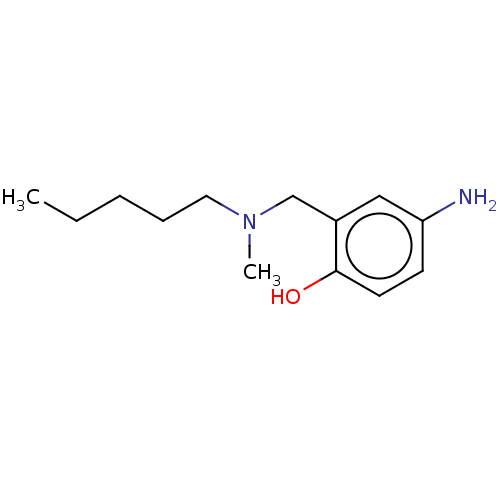
(CHEMBL4283986)Show InChI InChI=1S/C13H22N2O/c1-3-4-5-8-15(2)10-11-9-12(14)6-7-13(11)16/h6-7,9,16H,3-5,8,10,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468988
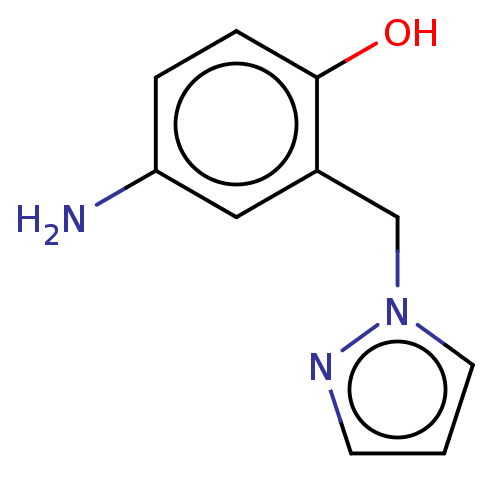
(CHEMBL4292399)Show InChI InChI=1S/C10H11N3O/c11-9-2-3-10(14)8(6-9)7-13-5-1-4-12-13/h1-6,14H,7,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468999
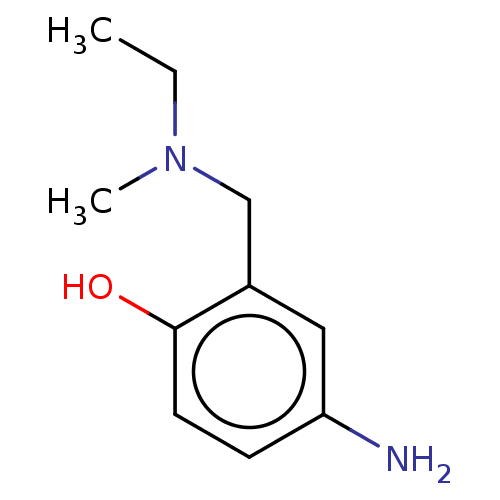
(CHEMBL4284261)Show InChI InChI=1S/C10H16N2O/c1-3-12(2)7-8-6-9(11)4-5-10(8)13/h4-6,13H,3,7,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468996
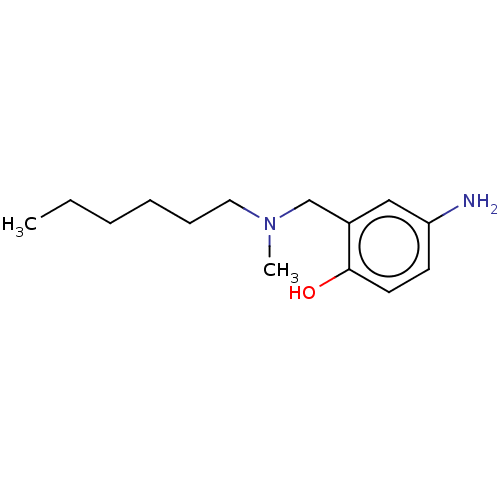
(CHEMBL4282360)Show InChI InChI=1S/C14H24N2O/c1-3-4-5-6-9-16(2)11-12-10-13(15)7-8-14(12)17/h7-8,10,17H,3-6,9,11,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468987

(CHEMBL4288742)Show InChI InChI=1S/C11H18N2O/c1-3-6-13(2)8-9-7-10(12)4-5-11(9)14/h4-5,7,14H,3,6,8,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.15E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50468992
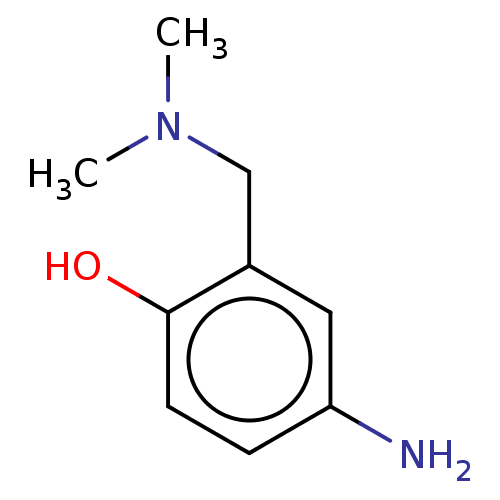
(CHEMBL4276921)Show InChI InChI=1S/C9H14N2O/c1-11(2)6-7-5-8(10)3-4-9(7)12/h3-5,12H,6,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.54E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Eur J Med Chem 157: 151-160 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.016
BindingDB Entry DOI: 10.7270/Q2FF3W3C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data