

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484478 (CHEMBL1929019 | jm5b01461, Compound 47) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484484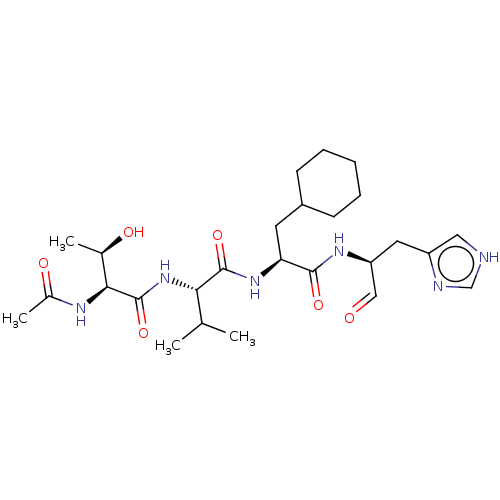 (CHEMBL1929023) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484483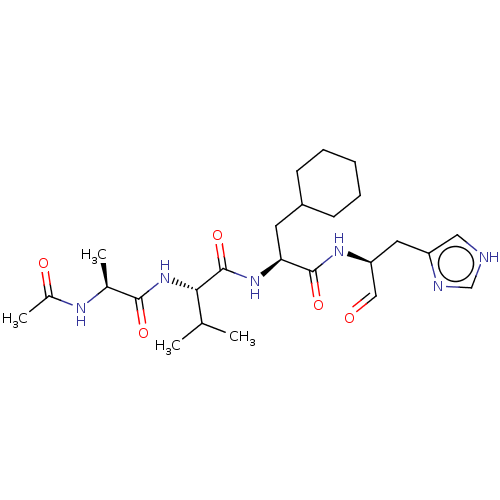 (CHEMBL1929020 | acs.jmedchem.1c00409_ST.132) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484491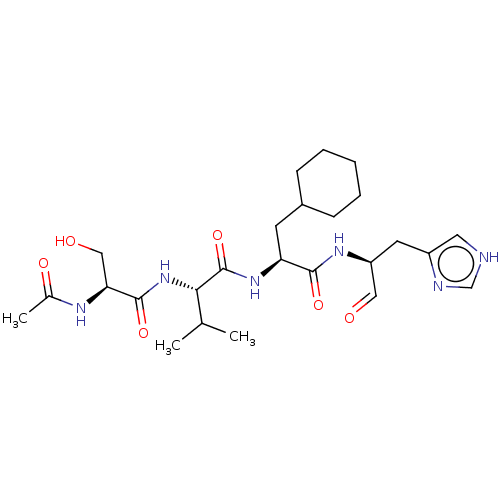 (CHEMBL1929022 | acs.jmedchem.1c00409_ST.144) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484487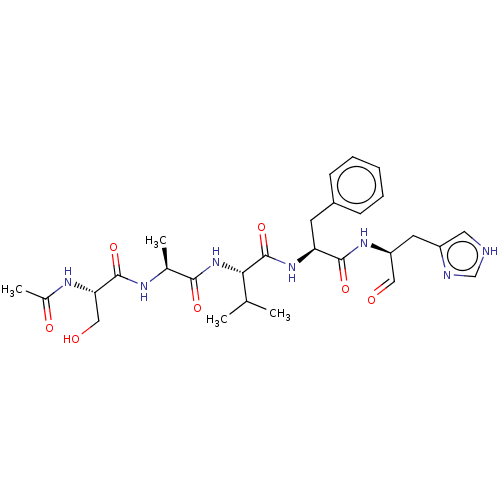 (CHEMBL1929018 | acs.jmedchem.1c00409_ST.150) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484482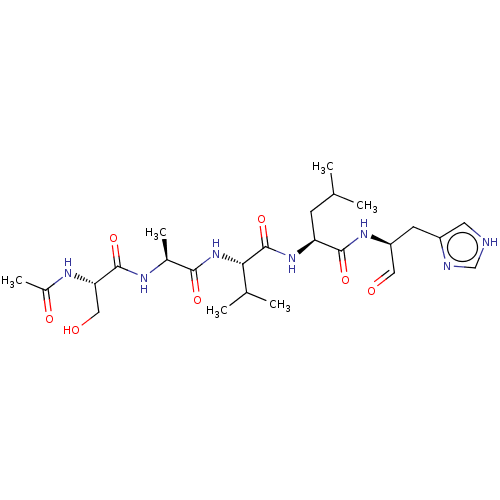 (CHEMBL1929017 | acs.jmedchem.1c00409_ST.380 | med....) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 preincubated for 20 mins before substrate addition and measured aft... | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484482 (CHEMBL1929017 | acs.jmedchem.1c00409_ST.380 | med....) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484490 (CHEMBL1929021 | acs.jmedchem.1c00409_ST.508) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484479 (CHEMBL479724 | acs.jmedchem.1c00409_ST.669 | med.2...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 preincubated for 20 mins before substrate addition and measured aft... | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484485 (CHEMBL1929016 | acs.jmedchem.1c00409_ST.700) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 preincubated for 20 mins before substrate addition and measured aft... | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484488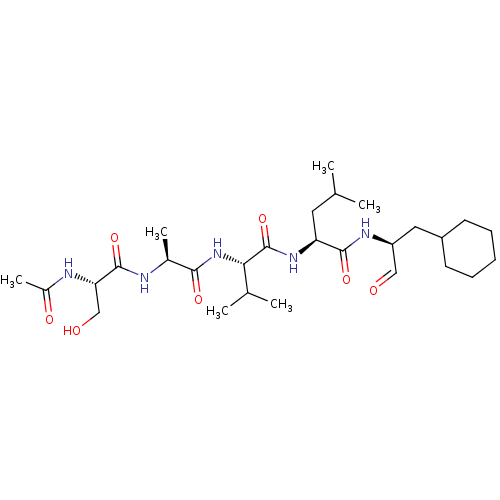 (CHEMBL1929015 | acs.jmedchem.1c00409_ST.720) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 preincubated for 20 mins before substrate addition and measured aft... | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484489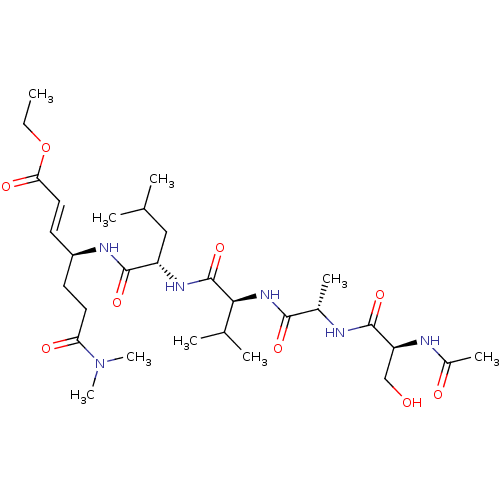 (CHEMBL1929009) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 preincubated for 20 mins before substrate addition and measured aft... | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484481 (CHEMBL1929014 | acs.jmedchem.1c00409_ST.815) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 preincubated for 20 mins before substrate addition and measured aft... | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484480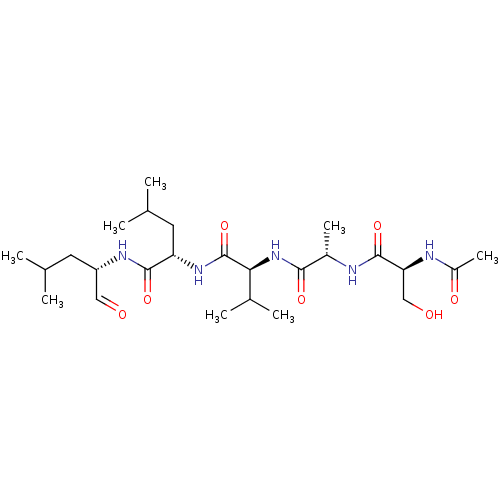 (CHEMBL1929012) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 preincubated for 20 mins before substrate addition and measured aft... | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484486 (CHEMBL1929013) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 preincubated for 20 mins before substrate addition and measured aft... | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||