

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50032523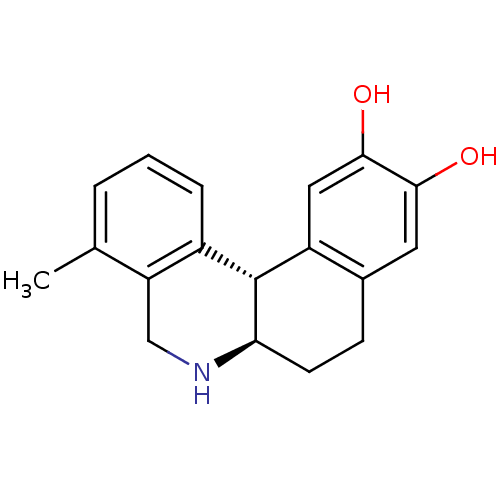 ((6aR,12bS)-4-Methyl-5,6,6a,7,8,12b-hexahydro-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 151 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50032520 ((6aR,12bS)-3-Methyl-5,6,6a,7,8,12b-hexahydro-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 134 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50010686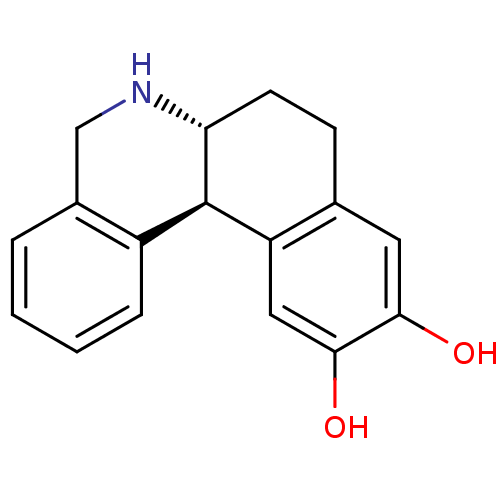 ((6aR,12bS)-10,11-Dihydroxy-5,6,6a,7,8,12b-hexahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50010684 ((6aR,12bS)-6-Propyl-5,6,6a,7,8,12b-hexahydro-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >10 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50032517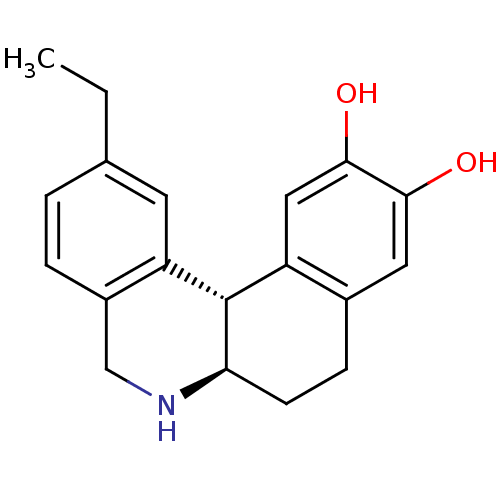 ((6aR,12bS)-2-Ethyl-5,6,6a,7,8,12b-hexahydro-benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50032518 ((6aR,12bS)-3-Methyl-6-propyl-5,6,6a,7,8,12b-hexahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50032516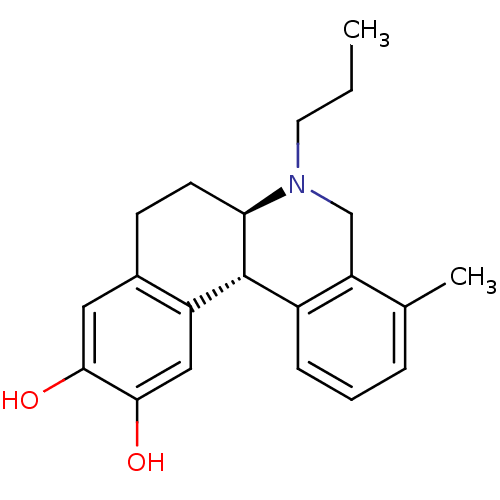 ((6aR,12bS)-4-Methyl-6-propyl-5,6,6a,7,8,12b-hexahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 333 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50032521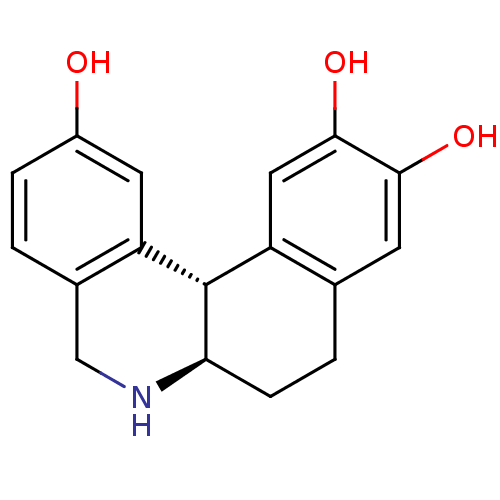 ((6aR,12bS)-5,6,6a,7,8,12b-Hexahydro-benzo[a]phenan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 148 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50032522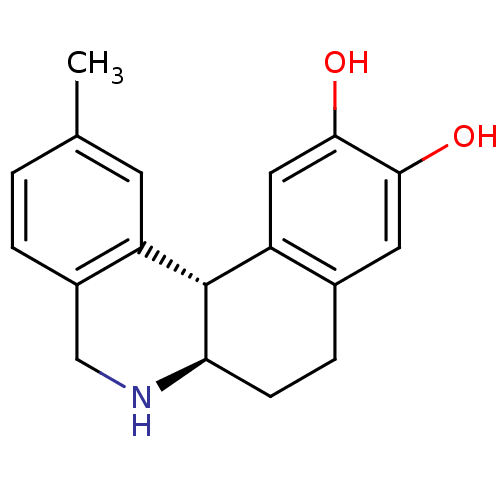 ((6aR,12bS)-2-Methyl-5,6,6a,7,8,12b-hexahydro-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 114 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50032524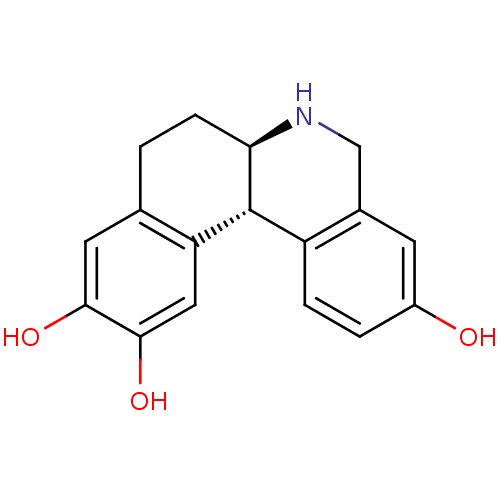 ((6aR,12bS)-5,6,6a,7,8,12b-Hexahydro-benzo[a]phenan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 5 (Rattus norvegicus) | BDBM50032519 ((6aR,12bS)-2-Phenyl-5,6,6a,7,8,12b-hexahydro-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Effective concentration of the compound as Adenylate cyclase activity was measured in rat homogenate | J Med Chem 38: 3062-70 (1995) BindingDB Entry DOI: 10.7270/Q2JD4VTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||