

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50033185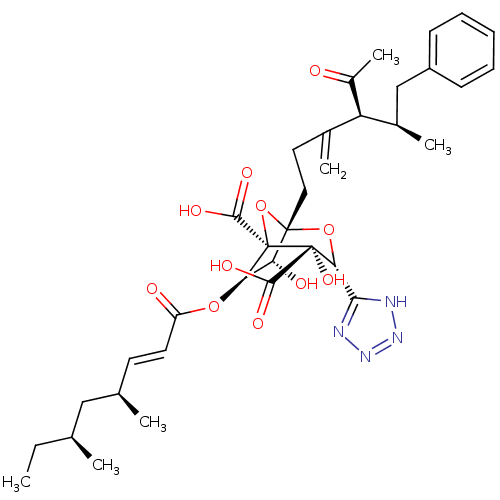 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033196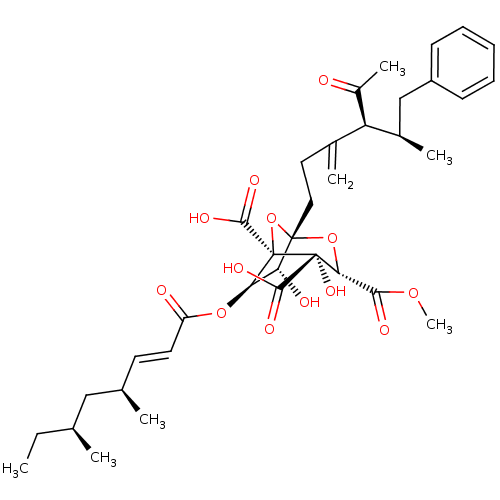 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033184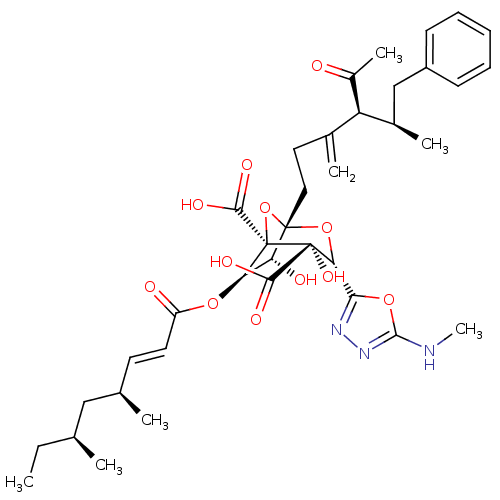 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033199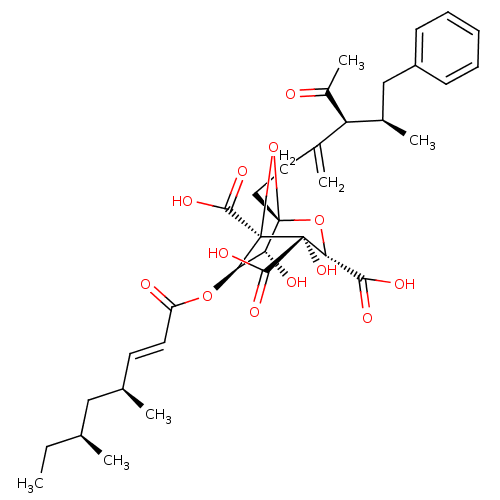 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033187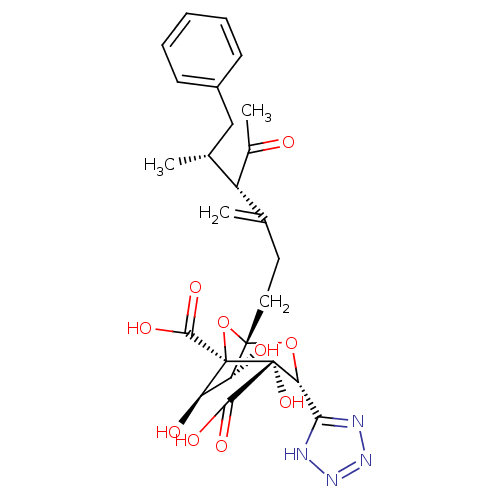 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033181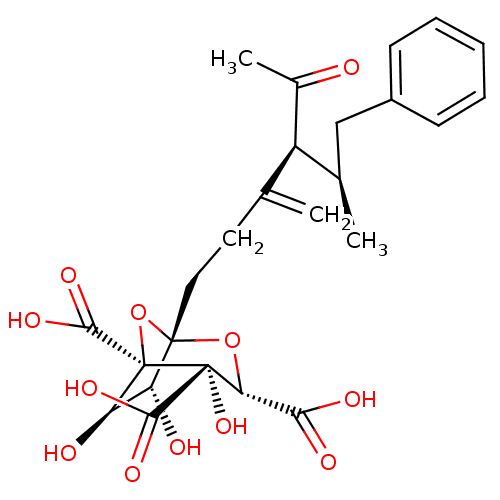 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033192 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033205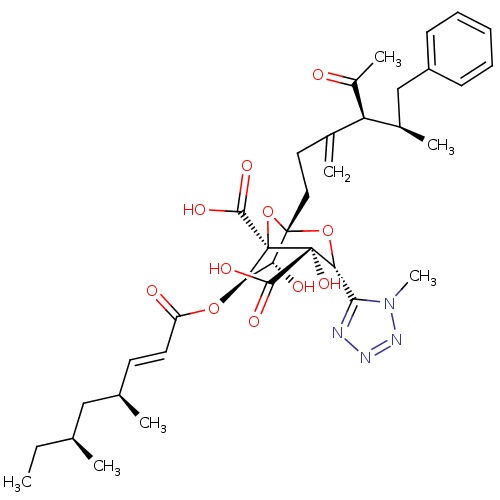 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033186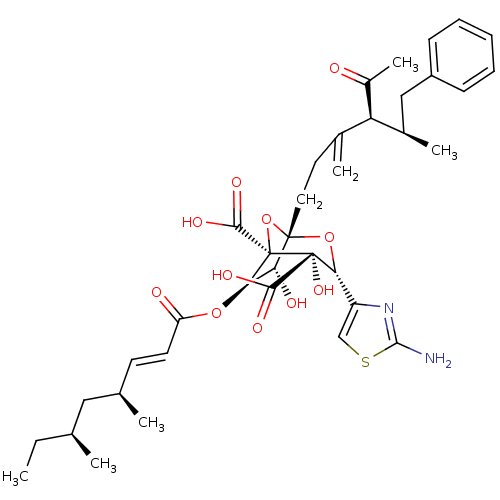 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033200 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033190 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033183 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033193 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033195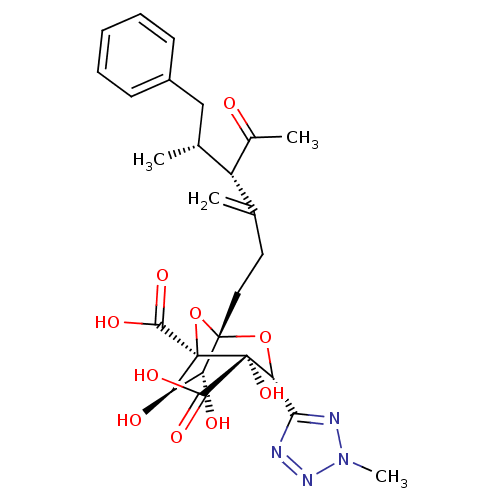 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033182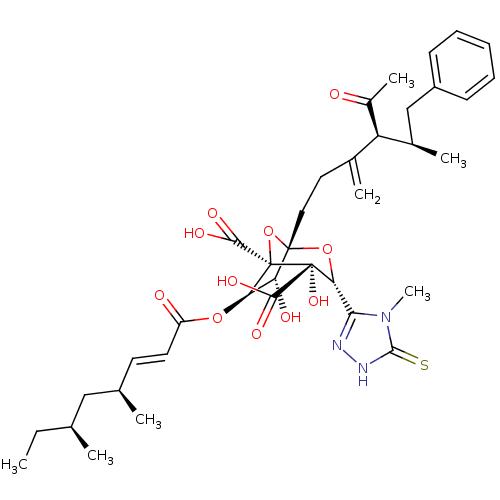 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033197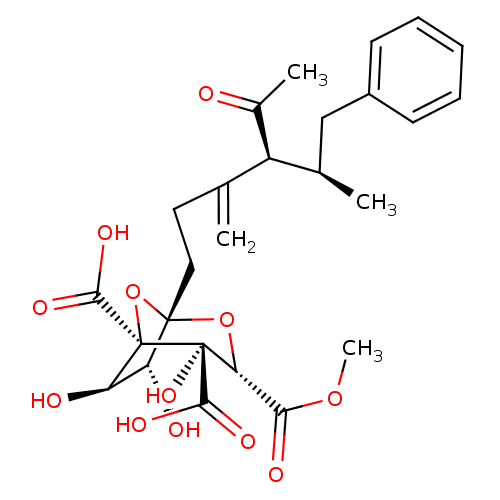 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033194 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033198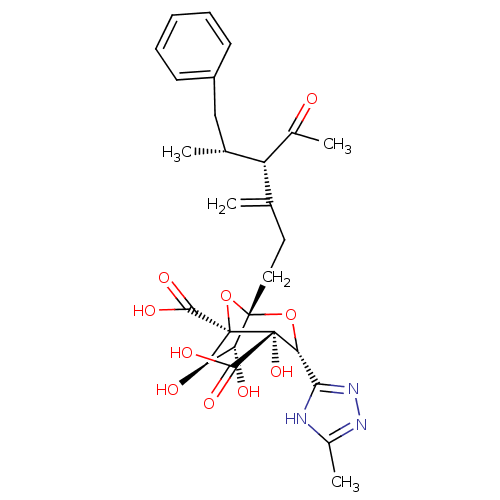 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033204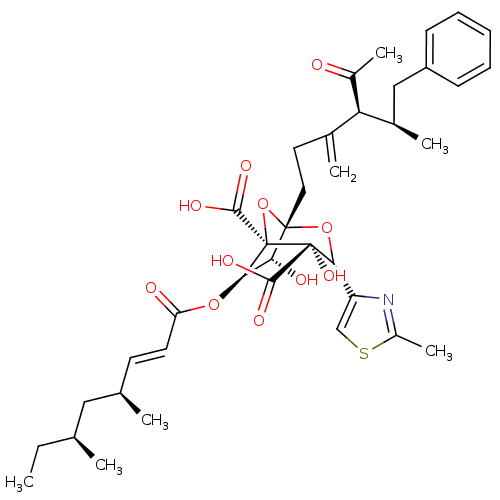 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033191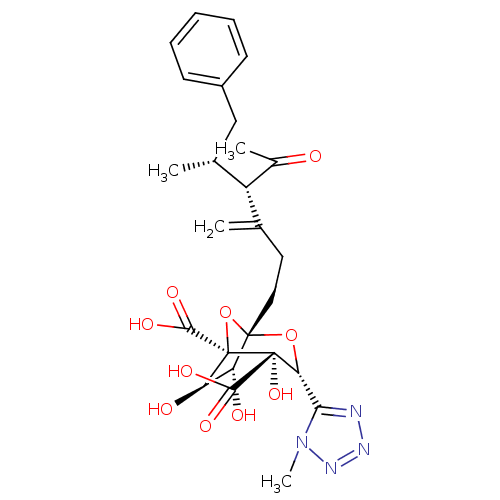 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 442 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033203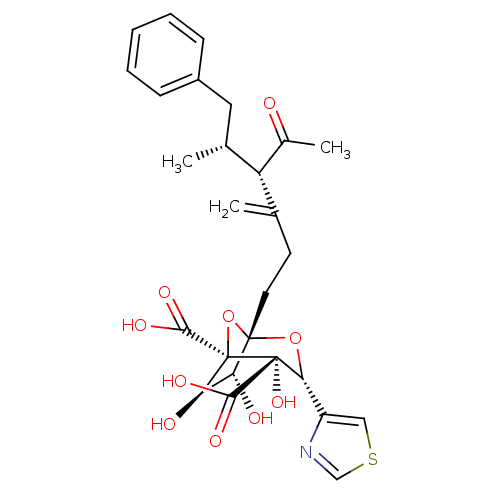 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033202 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 785 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033188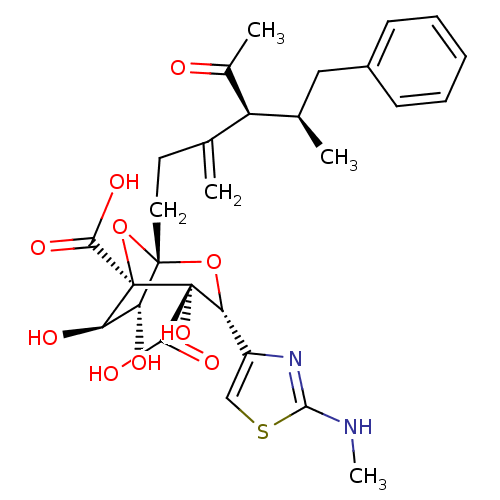 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033201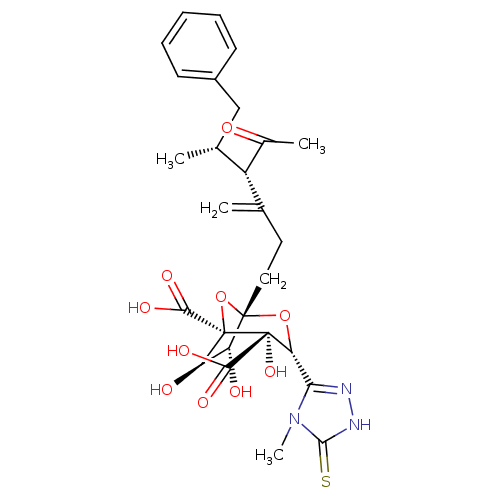 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50033189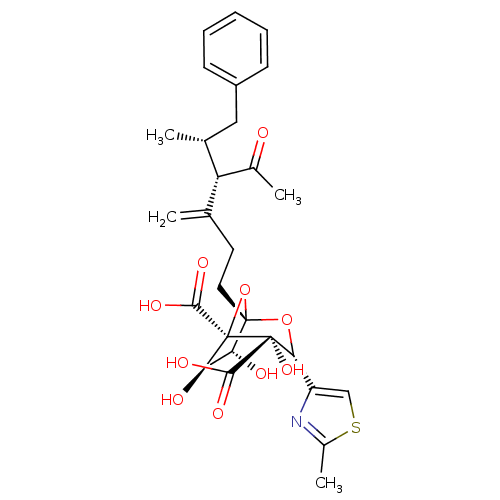 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetyl-5-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro for inhibitory activity against Squalene synthase in rat | J Med Chem 38: 3502-13 (1995) BindingDB Entry DOI: 10.7270/Q2M61J99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||