

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50260529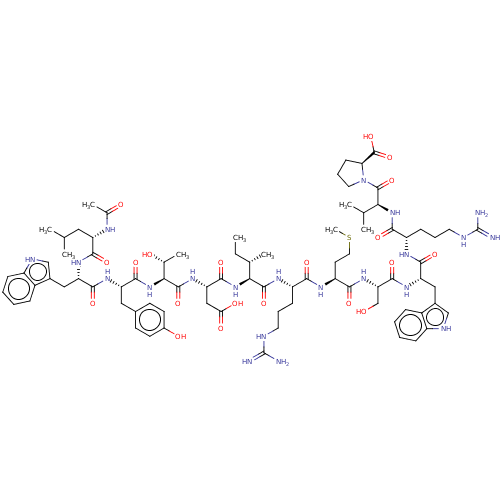 (CHEMBL4077626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description Opioid receptor mu 1 affinity against the receptor site model site 1 (mu1) by using the curve-fitting program LIGAND | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239363 (CHEMBL4094351) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50602185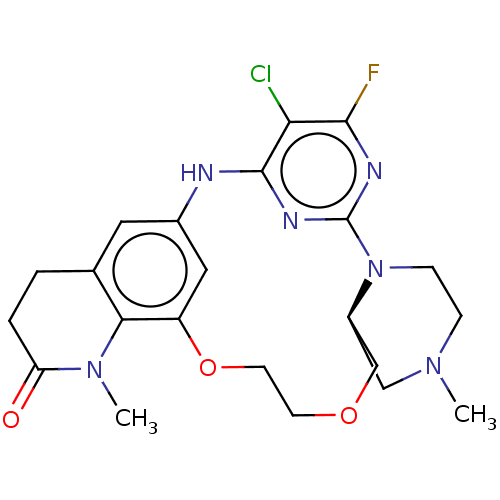 (CHEMBL5184651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239362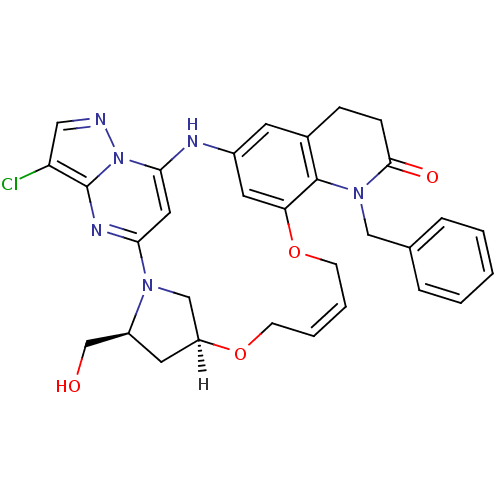 (CHEMBL4064865) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50391222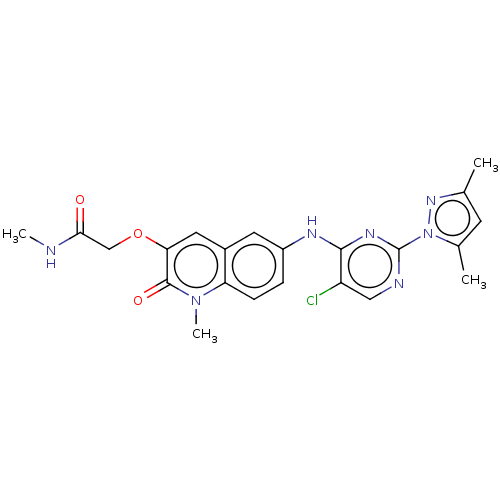 (CHEMBL5286830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239385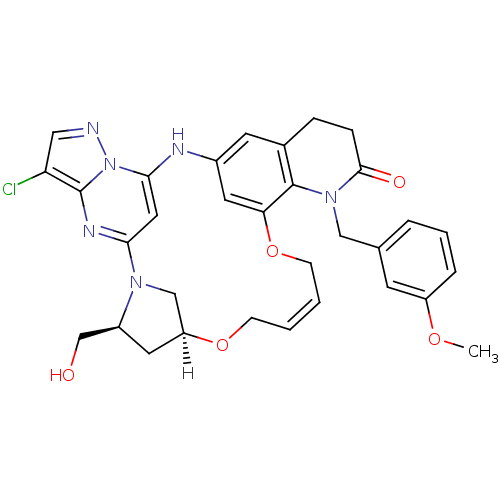 (CHEMBL4092565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50230693 (CHEMBL5269254) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description Opioid receptor affinity against Opioid receptor kappa 1 by using the curve-fitting program LIGAND | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50391220 (CHEMBL5277976) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50391221 (CHEMBL5273385) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50230700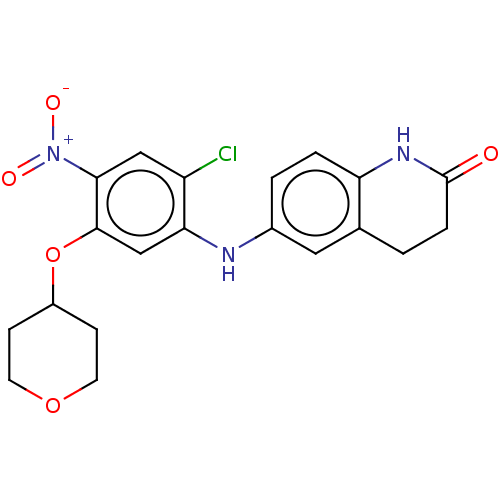 (CHEMBL4075278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239385 (CHEMBL4092565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50602186 (CHEMBL5207356) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50230699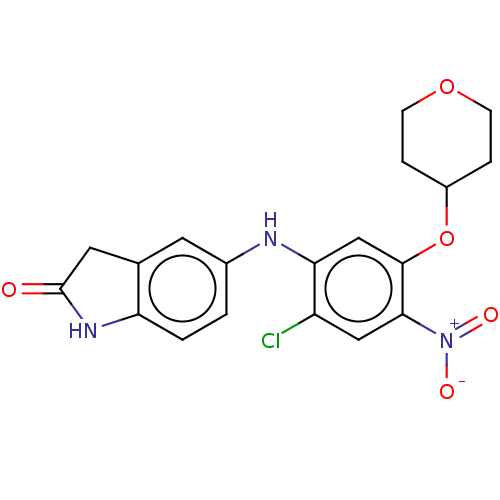 (CHEMBL4076344) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50541994 (CHEMBL4646073) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239362 (CHEMBL4064865) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50541993 (CHEMBL4640734) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50230692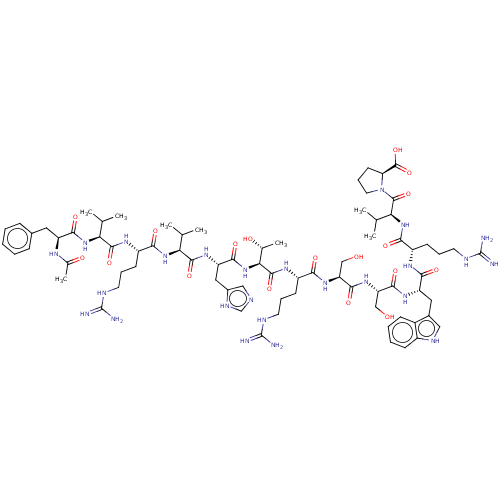 (CHEMBL5287879) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description Opioid receptor mu 1 affinity against the receptor site model site 1 (mu1) | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50508949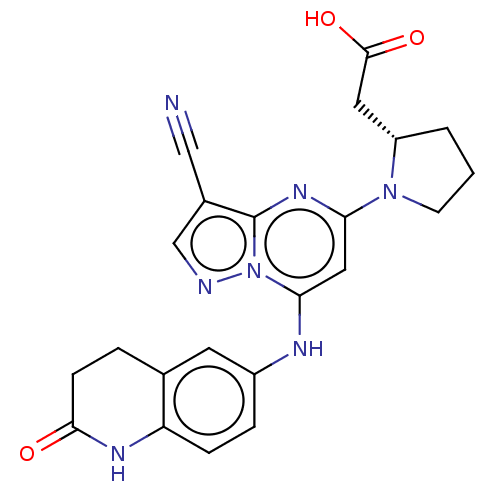 (CHEMBL4061857) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50541995 (CHEMBL4644523) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50508943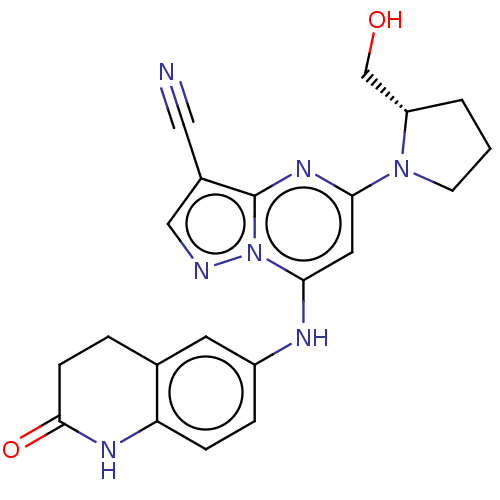 (CHEMBL4077836) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50511632 (CHEMBL4445645) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Casein kinase II subunit alpha 3 (Homo sapiens) | BDBM50239363 (CHEMBL4094351) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50511642 (CHEMBL4547183) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50511652 (CHEMBL4572689) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239363 (CHEMBL4094351) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50230696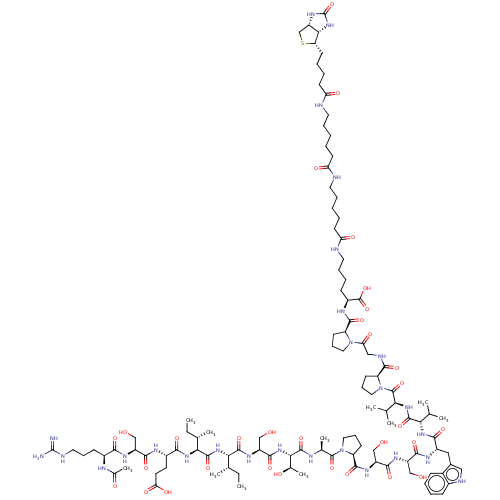 (CHEMBL5277214) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description The compound was evaluated for the antagonistic activity against beta-1 adrenergic receptor | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50391221 (CHEMBL5273385) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50511612 (CHEMBL4542555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50391220 (CHEMBL5277976) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor was measured by the inhibition of the positive chronotropic effectof isoproterenolin ... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50602186 (CHEMBL5207356) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha 3 (Homo sapiens) | BDBM50239362 (CHEMBL4064865) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha 3 (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha 3 (Homo sapiens) | BDBM50239385 (CHEMBL4092565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50511612 (CHEMBL4542555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50546199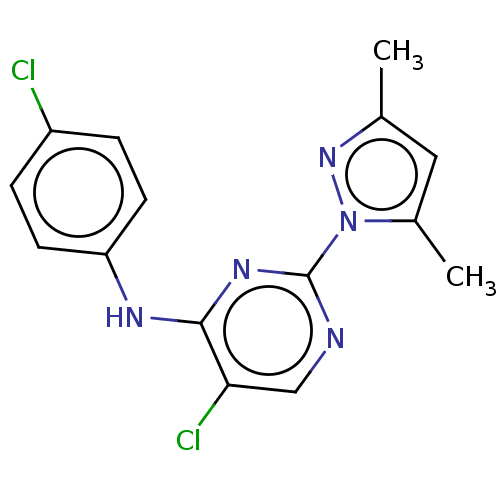 (CHEMBL4751268) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50382931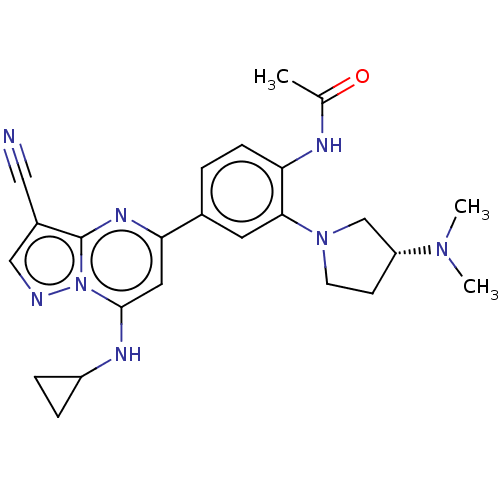 (CHEMBL4065661) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50511647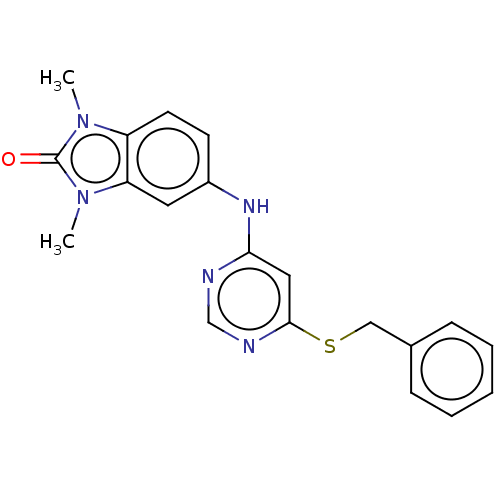 (CHEMBL4581204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50511633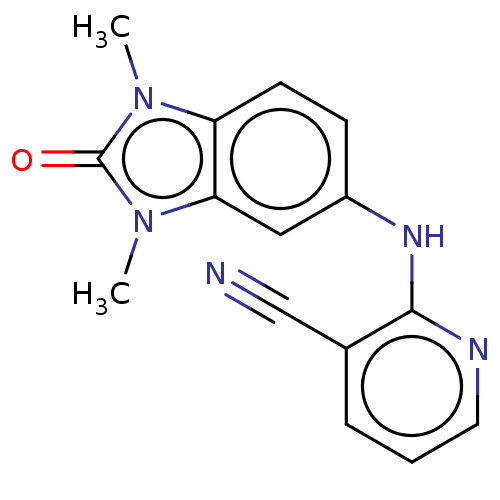 (CHEMBL4471490) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50511649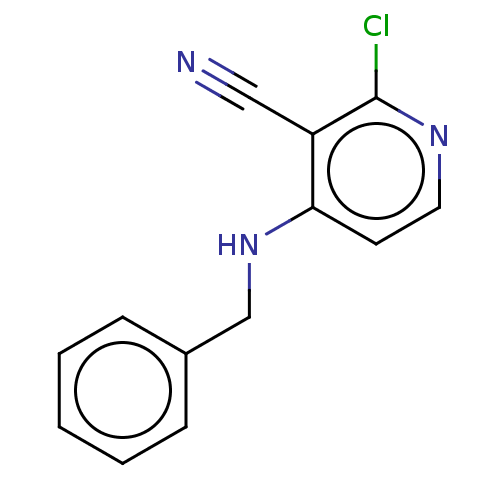 (CHEMBL1727323) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50230695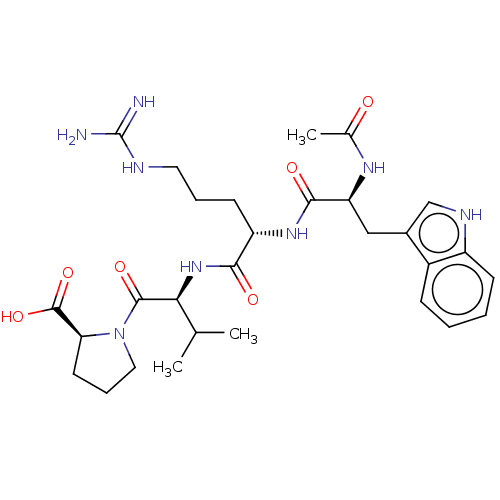 (CHEMBL5275452) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description The compound was evaluated for the antagonistic activity against beta-1 adrenergic receptor. | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50511611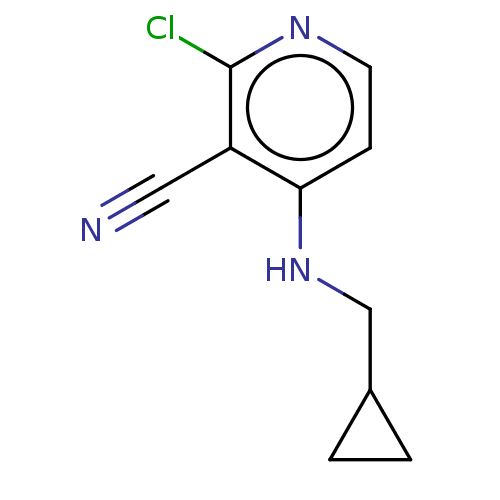 (CHEMBL4464160) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50230697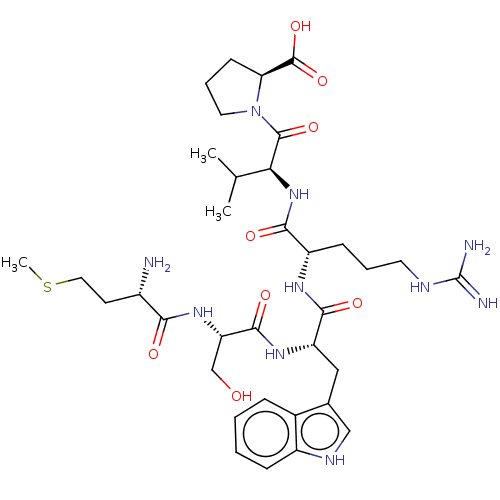 (CHEMBL5270561) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description The compound was evaluated for the antagonistic activity against beta-1 adrenergic receptor. | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50602184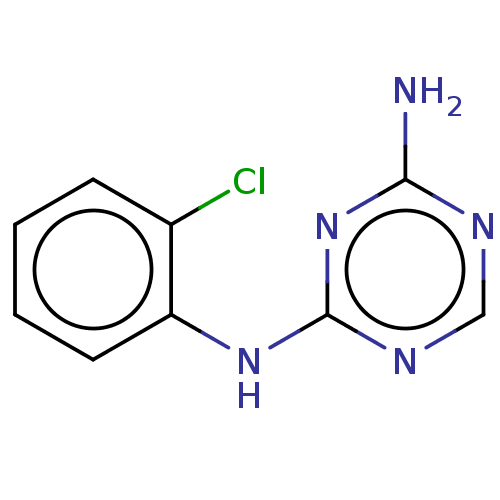 (CHEMBL1721570) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50230698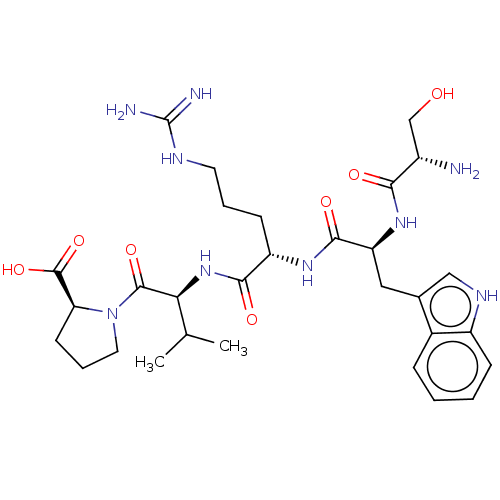 (CHEMBL5267389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50266782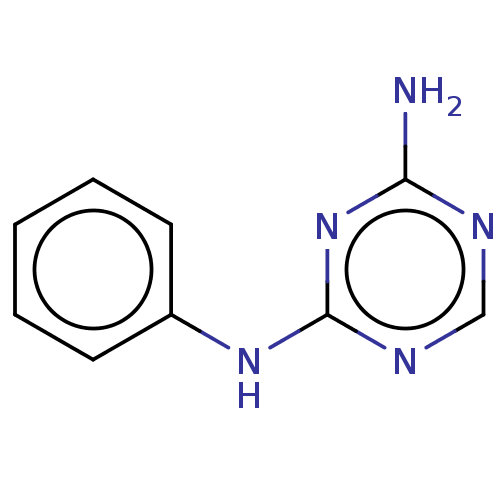 (Amanozine | Amenozine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 1.20E+6 | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50260529 (CHEMBL4077626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50292588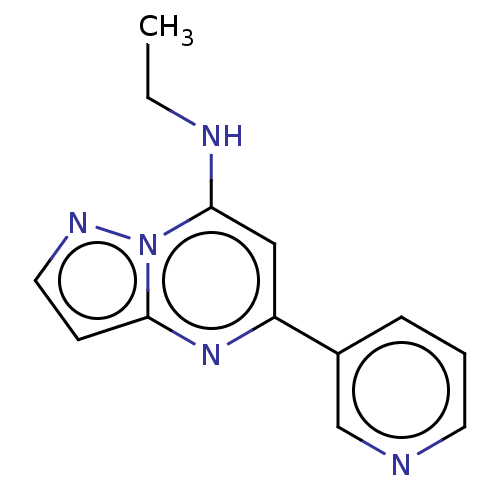 (CHEMBL4071527) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 6.90E+5 | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50508949 (CHEMBL4061857) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA Curated by ChEMBL | Assay Description In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... | J Med Chem 39: 2197-206 (1996) Article DOI: 10.1021/jm9508853 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 60 total ) | Next | Last >> |