 Found 42 hits Enz. Inhib. hit(s) with all data for entry = 50006912
Found 42 hits Enz. Inhib. hit(s) with all data for entry = 50006912 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50051317

((3-Chloro-phenyl)-(5,6-dimethyl-7H-pyrrolo[2,3-d]p...)Show InChI InChI=1S/C14H13ClN4/c1-8-9(2)18-13-12(8)14(17-7-16-13)19-11-5-3-4-10(15)6-11/h3-7H,1-2H3,(H2,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50051297
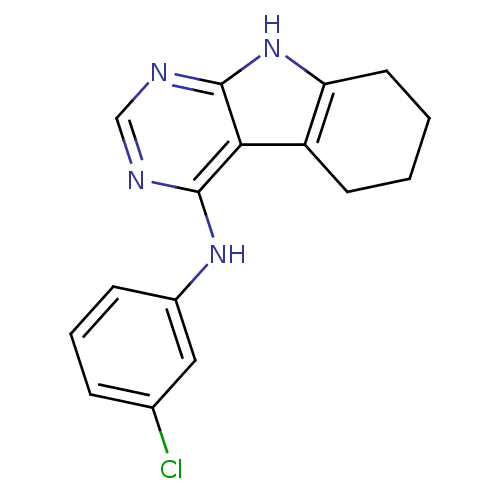
((3-Chloro-phenyl)-(6,7,8,9-tetrahydro-5H-pyrimido[...)Show InChI InChI=1S/C16H15ClN4/c17-10-4-3-5-11(8-10)20-15-14-12-6-1-2-7-13(12)21-16(14)19-9-18-15/h3-5,8-9H,1-2,6-7H2,(H2,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50504653
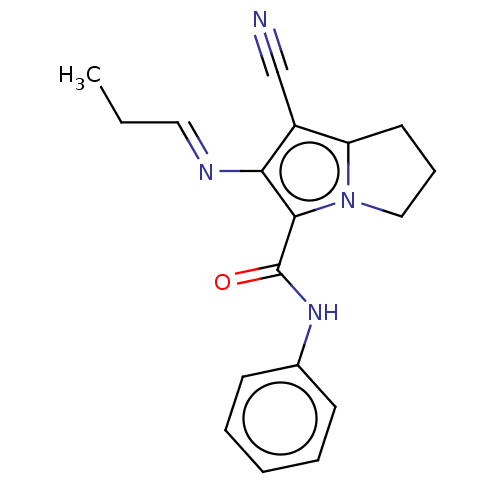
(CHEMBL4527978)Show InChI InChI=1S/C18H18N4O/c1-2-10-20-16-14(12-19)15-9-6-11-22(15)17(16)18(23)21-13-7-4-3-5-8-13/h3-5,7-8,10H,2,6,9,11H2,1H3,(H,21,23)/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) incubated for 40 mins by ADP-glo assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50504651

(CHEMBL4471323)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCn2c1C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H20N4O2S/c1-2-17-24(16(25)12-27-17)18-14(11-21)15-9-6-10-23(15)19(18)20(26)22-13-7-4-3-5-8-13/h3-5,7-8,17H,2,6,9-10,12H2,1H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) incubated for 40 mins by ADP-glo assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504639

(CHEMBL4557315)Show SMILES Oc1ccc(\C=N\c2c(C#N)c3CCCn3c2C(=O)Nc2ccc(Br)cc2)cc1 Show InChI InChI=1S/C22H17BrN4O2/c23-15-5-7-16(8-6-15)26-22(29)21-20(18(12-24)19-2-1-11-27(19)21)25-13-14-3-9-17(28)10-4-14/h3-10,13,28H,1-2,11H2,(H,26,29)/b25-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504640

(CHEMBL4528233)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCn2c1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C20H19ClN4O2S/c1-2-17-25(16(26)11-28-17)18-14(10-22)15-4-3-9-24(15)19(18)20(27)23-13-7-5-12(21)6-8-13/h5-8,17H,2-4,9,11H2,1H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504647
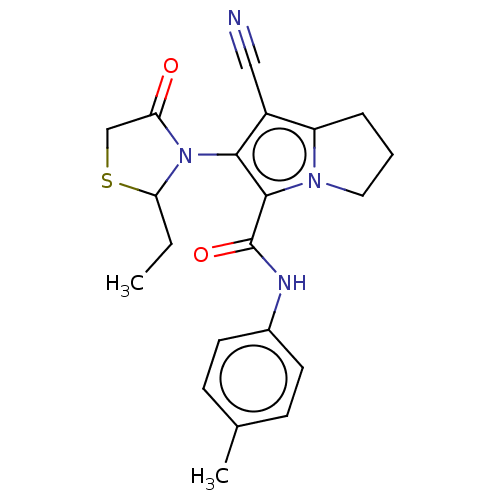
(CHEMBL4466133)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCn2c1C(=O)Nc1ccc(C)cc1 Show InChI InChI=1S/C21H22N4O2S/c1-3-18-25(17(26)12-28-18)19-15(11-22)16-5-4-10-24(16)20(19)21(27)23-14-8-6-13(2)7-9-14/h6-9,18H,3-5,10,12H2,1-2H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50504642
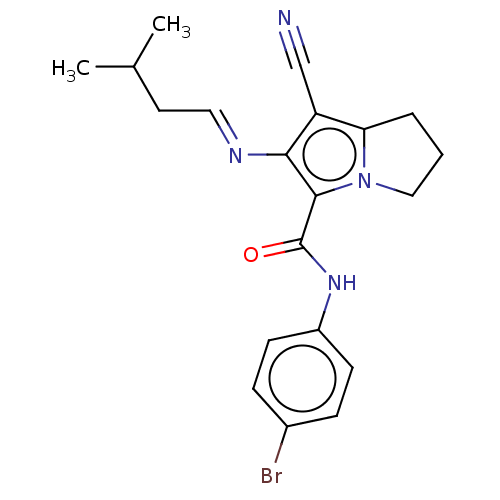
(CHEMBL4592358)Show SMILES CC(C)C\C=N\c1c(C#N)c2CCCn2c1C(=O)Nc1ccc(Br)cc1 Show InChI InChI=1S/C20H21BrN4O/c1-13(2)9-10-23-18-16(12-22)17-4-3-11-25(17)19(18)20(26)24-15-7-5-14(21)6-8-15/h5-8,10,13H,3-4,9,11H2,1-2H3,(H,24,26)/b23-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) incubated for 40 mins by ADP-glo assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504643
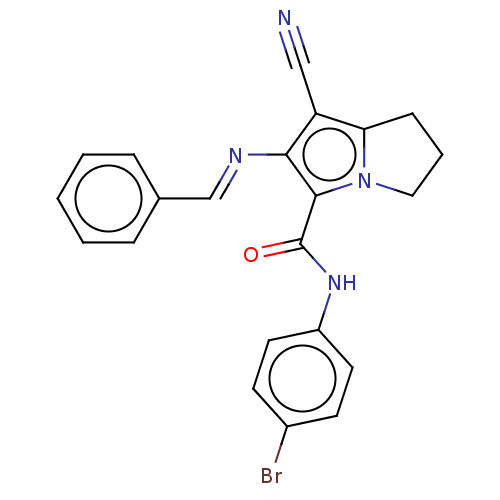
(CHEMBL4452428)Show SMILES Brc1ccc(NC(=O)c2c(\N=C\c3ccccc3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C22H17BrN4O/c23-16-8-10-17(11-9-16)26-22(28)21-20(25-14-15-5-2-1-3-6-15)18(13-24)19-7-4-12-27(19)21/h1-3,5-6,8-11,14H,4,7,12H2,(H,26,28)/b25-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504644
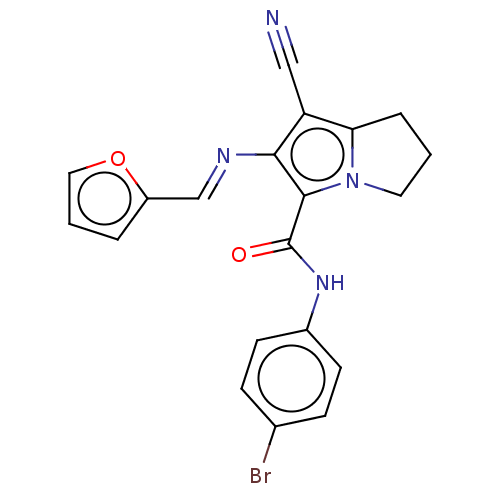
(CHEMBL4454216)Show SMILES Brc1ccc(NC(=O)c2c(\N=C\c3ccco3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C20H15BrN4O2/c21-13-5-7-14(8-6-13)24-20(26)19-18(23-12-15-3-2-10-27-15)16(11-22)17-4-1-9-25(17)19/h2-3,5-8,10,12H,1,4,9H2,(H,24,26)/b23-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504638
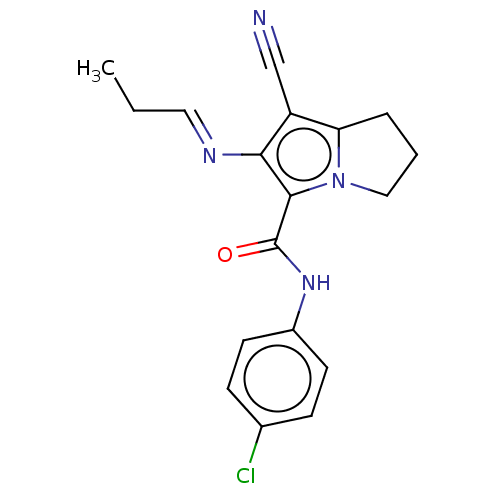
(CHEMBL4471314)Show InChI InChI=1S/C18H17ClN4O/c1-2-9-21-16-14(11-20)15-4-3-10-23(15)17(16)18(24)22-13-7-5-12(19)6-8-13/h5-9H,2-4,10H2,1H3,(H,22,24)/b21-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504648
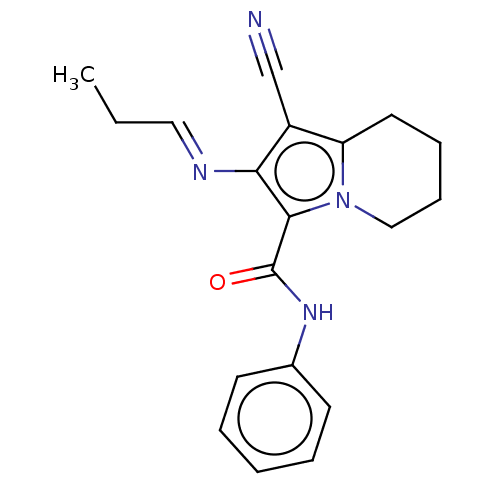
(CHEMBL4547592)Show InChI InChI=1S/C19H20N4O/c1-2-11-21-17-15(13-20)16-10-6-7-12-23(16)18(17)19(24)22-14-8-4-3-5-9-14/h3-5,8-9,11H,2,6-7,10,12H2,1H3,(H,22,24)/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504649

(CHEMBL4451436)Show SMILES Clc1ccc(\C=N\c2c(C#N)c3CCCn3c2C(=O)Nc2ccc(Br)cc2)cc1 Show InChI InChI=1S/C22H16BrClN4O/c23-15-5-9-17(10-6-15)27-22(29)21-20(18(12-25)19-2-1-11-28(19)21)26-13-14-3-7-16(24)8-4-14/h3-10,13H,1-2,11H2,(H,27,29)/b26-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504645
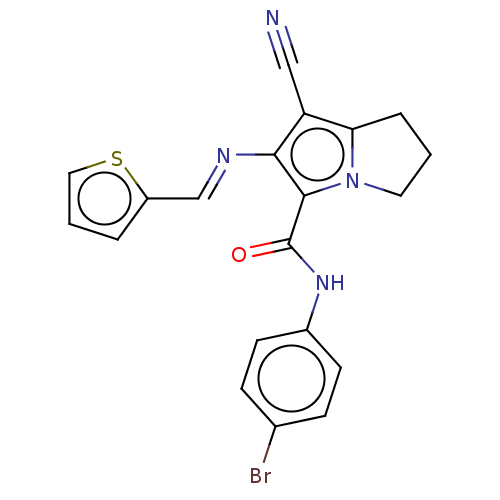
(CHEMBL4560186)Show SMILES Brc1ccc(NC(=O)c2c(\N=C\c3cccs3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C20H15BrN4OS/c21-13-5-7-14(8-6-13)24-20(26)19-18(23-12-15-3-2-10-27-15)16(11-22)17-4-1-9-25(17)19/h2-3,5-8,10,12H,1,4,9H2,(H,24,26)/b23-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504646

(CHEMBL4531924)Show SMILES Brc1ccc(NC(=O)c2c(\N=C\c3cccnc3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C21H16BrN5O/c22-15-5-7-16(8-6-15)26-21(28)20-19(25-13-14-3-1-9-24-12-14)17(11-23)18-4-2-10-27(18)20/h1,3,5-9,12-13H,2,4,10H2,(H,26,28)/b25-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504650
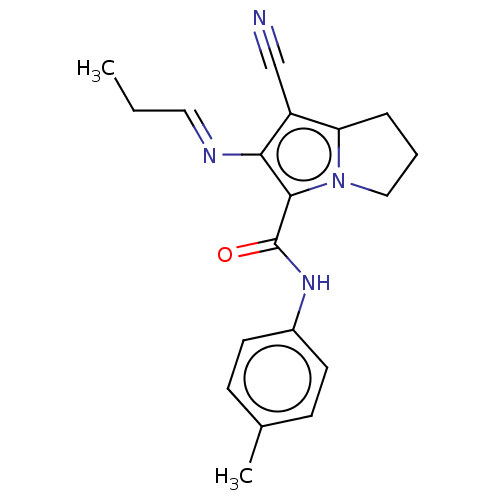
(CHEMBL4574444)Show InChI InChI=1S/C19H20N4O/c1-3-10-21-17-15(12-20)16-5-4-11-23(16)18(17)19(24)22-14-8-6-13(2)7-9-14/h6-10H,3-5,11H2,1-2H3,(H,22,24)/b21-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504651

(CHEMBL4471323)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCn2c1C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H20N4O2S/c1-2-17-24(16(25)12-27-17)18-14(11-21)15-9-6-10-23(15)19(18)20(26)22-13-7-4-3-5-8-13/h3-5,7-8,17H,2,6,9-10,12H2,1H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504644

(CHEMBL4454216)Show SMILES Brc1ccc(NC(=O)c2c(\N=C\c3ccco3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C20H15BrN4O2/c21-13-5-7-14(8-6-13)24-20(26)19-18(23-12-15-3-2-10-27-15)16(11-22)17-4-1-9-25(17)19/h2-3,5-8,10,12H,1,4,9H2,(H,24,26)/b23-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504641

(CHEMBL4460448)Show InChI InChI=1S/C18H17BrN4O/c1-2-9-21-16-14(11-20)15-4-3-10-23(15)17(16)18(24)22-13-7-5-12(19)6-8-13/h5-9H,2-4,10H2,1H3,(H,22,24)/b21-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504642

(CHEMBL4592358)Show SMILES CC(C)C\C=N\c1c(C#N)c2CCCn2c1C(=O)Nc1ccc(Br)cc1 Show InChI InChI=1S/C20H21BrN4O/c1-13(2)9-10-23-18-16(12-22)17-4-3-11-25(17)19(18)20(26)24-15-7-5-14(21)6-8-15/h5-8,10,13H,3-4,9,11H2,1-2H3,(H,24,26)/b23-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504641

(CHEMBL4460448)Show InChI InChI=1S/C18H17BrN4O/c1-2-9-21-16-14(11-20)15-4-3-10-23(15)17(16)18(24)22-13-7-5-12(19)6-8-13/h5-9H,2-4,10H2,1H3,(H,22,24)/b21-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504638

(CHEMBL4471314)Show InChI InChI=1S/C18H17ClN4O/c1-2-9-21-16-14(11-20)15-4-3-10-23(15)17(16)18(24)22-13-7-5-12(19)6-8-13/h5-9H,2-4,10H2,1H3,(H,22,24)/b21-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504648

(CHEMBL4547592)Show InChI InChI=1S/C19H20N4O/c1-2-11-21-17-15(13-20)16-10-6-7-12-23(16)18(17)19(24)22-14-8-4-3-5-9-14/h3-5,8-9,11H,2,6-7,10,12H2,1H3,(H,22,24)/b21-11+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504650

(CHEMBL4574444)Show InChI InChI=1S/C19H20N4O/c1-3-10-21-17-15(12-20)16-5-4-11-23(16)18(17)19(24)22-14-8-6-13(2)7-9-14/h6-10H,3-5,11H2,1-2H3,(H,22,24)/b21-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504651

(CHEMBL4471323)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCn2c1C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H20N4O2S/c1-2-17-24(16(25)12-27-17)18-14(11-21)15-9-6-10-23(15)19(18)20(26)22-13-7-4-3-5-8-13/h3-5,7-8,17H,2,6,9-10,12H2,1H3,(H,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504653

(CHEMBL4527978)Show InChI InChI=1S/C18H18N4O/c1-2-10-20-16-14(12-19)15-9-6-11-22(15)17(16)18(23)21-13-7-4-3-5-8-13/h3-5,7-8,10H,2,6,9,11H2,1H3,(H,21,23)/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504652
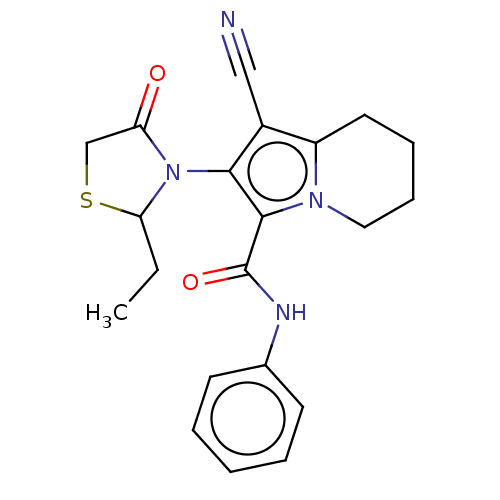
(CHEMBL4579212)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCCn2c1C(=O)Nc1ccccc1 Show InChI InChI=1S/C21H22N4O2S/c1-2-18-25(17(26)13-28-18)19-15(12-22)16-10-6-7-11-24(16)20(19)21(27)23-14-8-4-3-5-9-14/h3-5,8-9,18H,2,6-7,10-11,13H2,1H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504652

(CHEMBL4579212)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCCn2c1C(=O)Nc1ccccc1 Show InChI InChI=1S/C21H22N4O2S/c1-2-18-25(17(26)13-28-18)19-15(12-22)16-10-6-7-11-24(16)20(19)21(27)23-14-8-4-3-5-9-14/h3-5,8-9,18H,2,6-7,10-11,13H2,1H3,(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 6.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504639

(CHEMBL4557315)Show SMILES Oc1ccc(\C=N\c2c(C#N)c3CCCn3c2C(=O)Nc2ccc(Br)cc2)cc1 Show InChI InChI=1S/C22H17BrN4O2/c23-15-5-7-16(8-6-15)26-22(29)21-20(18(12-24)19-2-1-11-27(19)21)25-13-14-3-9-17(28)10-4-14/h3-10,13,28H,1-2,11H2,(H,26,29)/b25-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) incubated for 40 mins by ADP-glo assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504653

(CHEMBL4527978)Show InChI InChI=1S/C18H18N4O/c1-2-10-20-16-14(12-19)15-9-6-11-22(15)17(16)18(23)21-13-7-4-3-5-8-13/h3-5,7-8,10H,2,6,9,11H2,1H3,(H,21,23)/b20-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504642

(CHEMBL4592358)Show SMILES CC(C)C\C=N\c1c(C#N)c2CCCn2c1C(=O)Nc1ccc(Br)cc1 Show InChI InChI=1S/C20H21BrN4O/c1-13(2)9-10-23-18-16(12-22)17-4-3-11-25(17)19(18)20(26)24-15-7-5-14(21)6-8-15/h5-8,10,13H,3-4,9,11H2,1-2H3,(H,24,26)/b23-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504643

(CHEMBL4452428)Show SMILES Brc1ccc(NC(=O)c2c(\N=C\c3ccccc3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C22H17BrN4O/c23-16-8-10-17(11-9-16)26-22(28)21-20(25-14-15-5-2-1-3-6-15)18(13-24)19-7-4-12-27(19)21/h1-3,5-6,8-11,14H,4,7,12H2,(H,26,28)/b25-14+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504645

(CHEMBL4560186)Show SMILES Brc1ccc(NC(=O)c2c(\N=C\c3cccs3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C20H15BrN4OS/c21-13-5-7-14(8-6-13)24-20(26)19-18(23-12-15-3-2-10-27-15)16(11-22)17-4-1-9-25(17)19/h2-3,5-8,10,12H,1,4,9H2,(H,24,26)/b23-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504646

(CHEMBL4531924)Show SMILES Brc1ccc(NC(=O)c2c(\N=C\c3cccnc3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C21H16BrN5O/c22-15-5-7-16(8-6-15)26-21(28)20-19(25-13-14-3-1-9-24-12-14)17(11-23)18-4-2-10-27(18)20/h1,3,5-9,12-13H,2,4,10H2,(H,26,28)/b25-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504647

(CHEMBL4466133)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCn2c1C(=O)Nc1ccc(C)cc1 Show InChI InChI=1S/C21H22N4O2S/c1-3-18-25(17(26)12-28-18)19-15(11-22)16-5-4-10-24(16)20(19)21(27)23-14-8-6-13(2)7-9-14/h6-9,18H,3-5,10,12H2,1-2H3,(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504640

(CHEMBL4528233)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCn2c1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C20H19ClN4O2S/c1-2-17-25(16(26)11-28-17)18-14(10-22)15-4-3-9-24(15)19(18)20(27)23-13-7-5-12(21)6-8-13/h5-8,17H,2-4,9,11H2,1H3,(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50504649

(CHEMBL4451436)Show SMILES Clc1ccc(\C=N\c2c(C#N)c3CCCn3c2C(=O)Nc2ccc(Br)cc2)cc1 Show InChI InChI=1S/C22H16BrClN4O/c23-15-5-9-17(10-6-15)27-22(29)21-20(18(12-25)19-2-1-11-28(19)21)26-13-14-3-7-16(24)8-4-14/h3-10,13H,1-2,11H2,(H,27,29)/b26-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data