 Found 51 hits Enz. Inhib. hit(s) with all data for entry = 50007315
Found 51 hits Enz. Inhib. hit(s) with all data for entry = 50007315 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061174
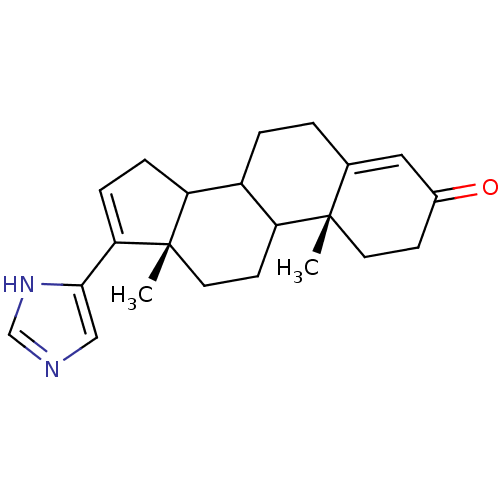
((10R,13S)-17-(1H-Imidazol-4-yl)-10,13-dimethyl-1,2...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cnc[nH]1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h6,11-13,16-18H,3-5,7-10H2,1-2H3,(H,23,24)/t16?,17?,18?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408273

(CHEMBL2112522)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ncc[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)13-14(21)3-4-16-17-5-6-19(20-23-11-12-24-20)22(17,2)10-8-18(16)21/h3,6,11-12,15-18,25H,4-5,7-10,13H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408267

(CHEMBL2112526)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1cnc[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h3,6,12-13,15-18,25H,4-5,7-11H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408271

(CHEMBL2112517)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccn[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-10-7-15(25)13-14(21)3-4-16-17-5-6-19(20-9-12-23-24-20)22(17,2)11-8-18(16)21/h3,6,9,12,15-18,25H,4-5,7-8,10-11,13H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061172
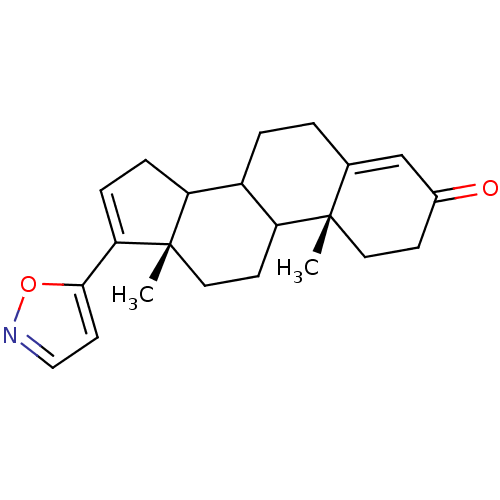
((10R,13S)-17-Isoxazol-5-yl-10,13-dimethyl-1,2,6,7,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1ccno1 |c:21,t:8| Show InChI InChI=1S/C22H27NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-23-25-20)22(17,2)11-8-18(16)21/h6,9,12-13,16-18H,3-5,7-8,10-11H2,1-2H3/t16?,17?,18?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061172

((10R,13S)-17-Isoxazol-5-yl-10,13-dimethyl-1,2,6,7,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1ccno1 |c:21,t:8| Show InChI InChI=1S/C22H27NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-23-25-20)22(17,2)11-8-18(16)21/h6,9,12-13,16-18H,3-5,7-8,10-11H2,1-2H3/t16?,17?,18?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408271

(CHEMBL2112517)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccn[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-10-7-15(25)13-14(21)3-4-16-17-5-6-19(20-9-12-23-24-20)22(17,2)11-8-18(16)21/h3,6,9,12,15-18,25H,4-5,7-8,10-11,13H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408267

(CHEMBL2112526)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1cnc[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h3,6,12-13,15-18,25H,4-5,7-11H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061174

((10R,13S)-17-(1H-Imidazol-4-yl)-10,13-dimethyl-1,2...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cnc[nH]1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h6,11-13,16-18H,3-5,7-10H2,1-2H3,(H,23,24)/t16?,17?,18?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061169

((10R,13S,17S)-17-(1H-Imidazol-4-yl)-10,13-dimethyl...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC[C@@H]2c1cnc[nH]1 |t:8| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h11-13,16-19H,3-10H2,1-2H3,(H,23,24)/t16?,17?,18?,19-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061185
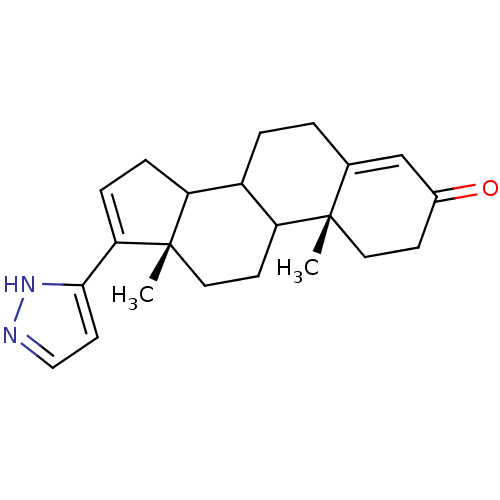
((10R,13S)-10,13-Dimethyl-17-(1H-pyrazol-3-yl)-1,2,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1ccn[nH]1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-10-7-15(25)13-14(21)3-4-16-17-5-6-19(20-9-12-23-24-20)22(17,2)11-8-18(16)21/h6,9,12-13,16-18H,3-5,7-8,10-11H2,1-2H3,(H,23,24)/t16?,17?,18?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408269
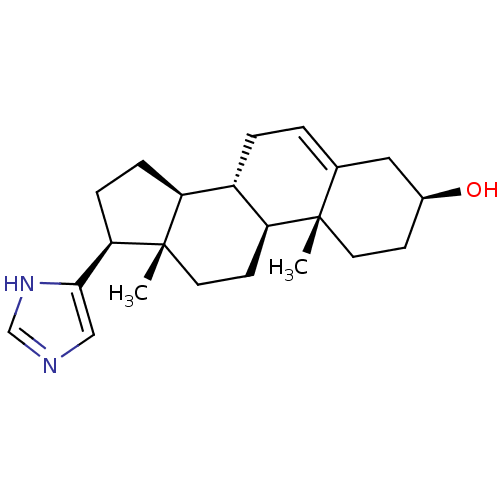
(CHEMBL2112518)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2c1cnc[nH]1 |t:7| Show InChI InChI=1S/C22H32N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h3,12-13,15-19,25H,4-11H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,19+,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408265
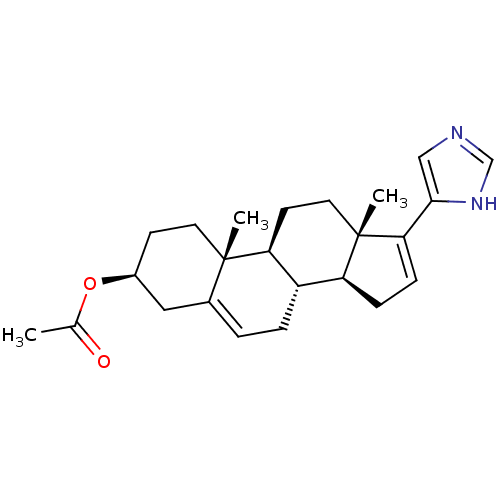
(CHEMBL2112534)Show SMILES CC(=O)O[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC=C4c4cnc[nH]4)[C@@H]3CC=C2C1 |c:16,28| Show InChI InChI=1S/C24H32N2O2/c1-15(27)28-17-8-10-23(2)16(12-17)4-5-18-19-6-7-21(22-13-25-14-26-22)24(19,3)11-9-20(18)23/h4,7,13-14,17-20H,5-6,8-12H2,1-3H3,(H,25,26)/t17-,18-,19-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061185

((10R,13S)-10,13-Dimethyl-17-(1H-pyrazol-3-yl)-1,2,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1ccn[nH]1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-10-7-15(25)13-14(21)3-4-16-17-5-6-19(20-9-12-23-24-20)22(17,2)11-8-18(16)21/h6,9,12-13,16-18H,3-5,7-8,10-11H2,1-2H3,(H,23,24)/t16?,17?,18?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061169

((10R,13S,17S)-17-(1H-Imidazol-4-yl)-10,13-dimethyl...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC[C@@H]2c1cnc[nH]1 |t:8| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h11-13,16-19H,3-10H2,1-2H3,(H,23,24)/t16?,17?,18?,19-,21+,22+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408272
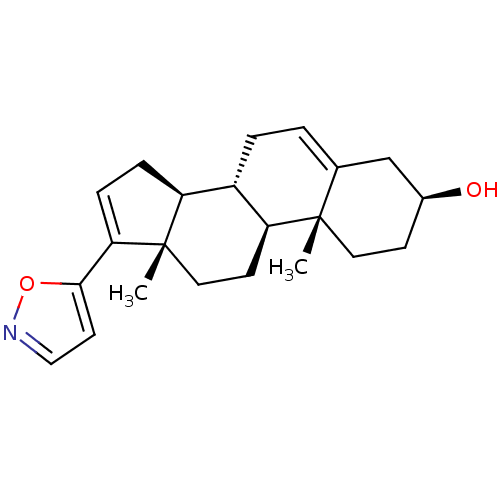
(CHEMBL2112521)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccno1 |c:21,t:7| Show InChI InChI=1S/C22H29NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-23-25-20)22(17,2)11-8-18(16)21/h3,6,9,12,15-18,24H,4-5,7-8,10-11,13H2,1-2H3/t15-,16-,17-,18-,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408269

(CHEMBL2112518)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2c1cnc[nH]1 |t:7| Show InChI InChI=1S/C22H32N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h3,12-13,15-19,25H,4-11H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,19+,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408268
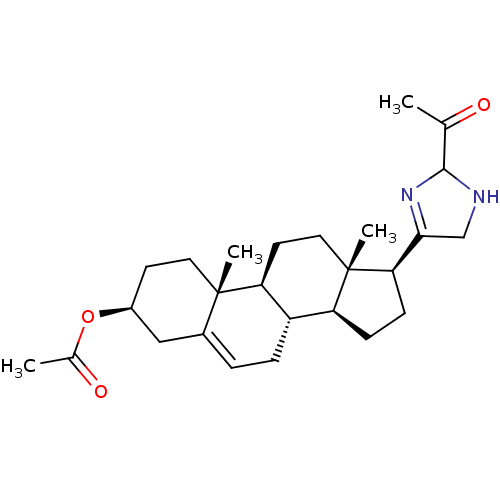
(CHEMBL2112524)Show SMILES CC(=O)O[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC[C@@H]4C4=NC(NC4)C(C)=O)[C@@H]3CC=C2C1 |c:31,t:19| Show InChI InChI=1S/C26H38N2O3/c1-15(29)24-27-14-23(28-24)22-8-7-20-19-6-5-17-13-18(31-16(2)30)9-11-25(17,3)21(19)10-12-26(20,22)4/h5,18-22,24,27H,6-14H2,1-4H3/t18-,19-,20-,21-,22+,24?,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408272

(CHEMBL2112521)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ccno1 |c:21,t:7| Show InChI InChI=1S/C22H29NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-23-25-20)22(17,2)11-8-18(16)21/h3,6,9,12,15-18,24H,4-5,7-8,10-11,13H2,1-2H3/t15-,16-,17-,18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061189
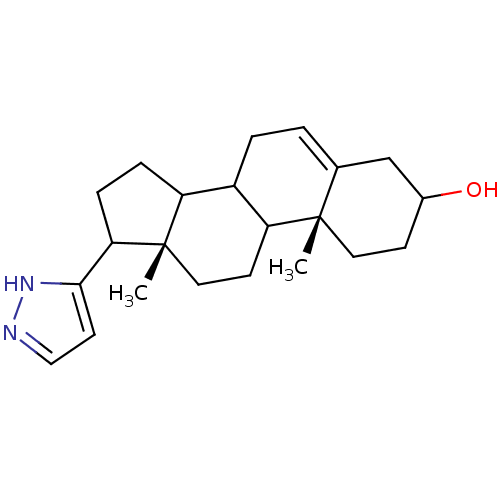
((10R,13S)-10,13-Dimethyl-17-(2H-pyrazol-3-yl)-2,3,...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CCC2c1ccn[nH]1 |t:7| Show InChI InChI=1S/C22H32N2O/c1-21-10-7-15(25)13-14(21)3-4-16-17-5-6-19(20-9-12-23-24-20)22(17,2)11-8-18(16)21/h3,9,12,15-19,25H,4-8,10-11,13H2,1-2H3,(H,23,24)/t15?,16?,17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061173
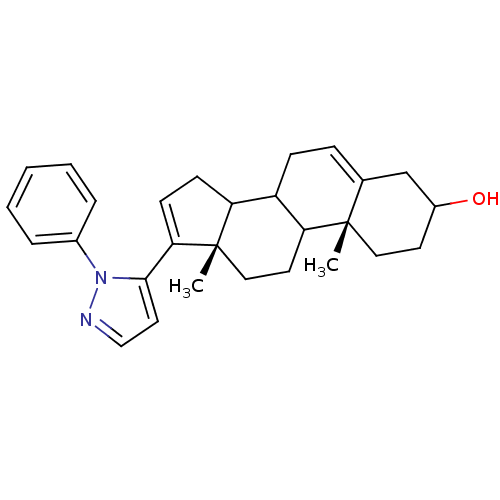
((10R,13S)-10,13-Dimethyl-17-(2-phenyl-2H-pyrazol-3...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CC=C2c1ccnn1-c1ccccc1 |c:21,t:7| Show InChI InChI=1S/C28H34N2O/c1-27-15-12-21(31)18-19(27)8-9-22-23-10-11-25(28(23,2)16-13-24(22)27)26-14-17-29-30(26)20-6-4-3-5-7-20/h3-8,11,14,17,21-24,31H,9-10,12-13,15-16,18H2,1-2H3/t21?,22?,23?,24?,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061181
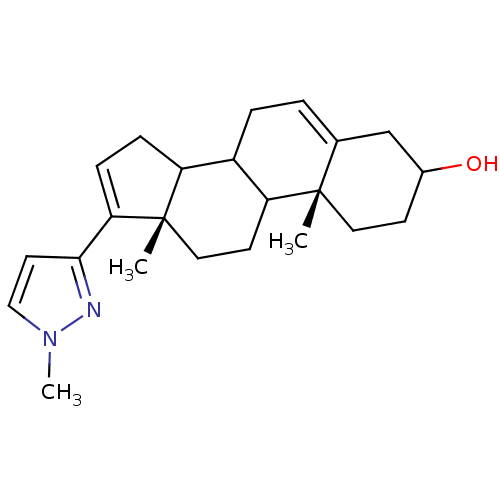
((10R,13S)-10,13-Dimethyl-17-(1-methyl-1H-pyrazol-3...)Show SMILES Cn1ccc(n1)C1=CCC2C3CC=C4CC(O)CC[C@]4(C)C3CC[C@]12C |t:7,13| Show InChI InChI=1S/C23H32N2O/c1-22-11-8-16(26)14-15(22)4-5-17-18-6-7-20(21-10-13-25(3)24-21)23(18,2)12-9-19(17)22/h4,7,10,13,16-19,26H,5-6,8-9,11-12,14H2,1-3H3/t16?,17?,18?,19?,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061186

((10R,13S)-17-Isoxazol-5-yl-10,13-dimethyl-2,3,4,7,...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CCC2c1ccno1 |t:7| Show InChI InChI=1S/C22H31NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-23-25-20)22(17,2)11-8-18(16)21/h3,9,12,15-19,24H,4-8,10-11,13H2,1-2H3/t15?,16?,17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061166
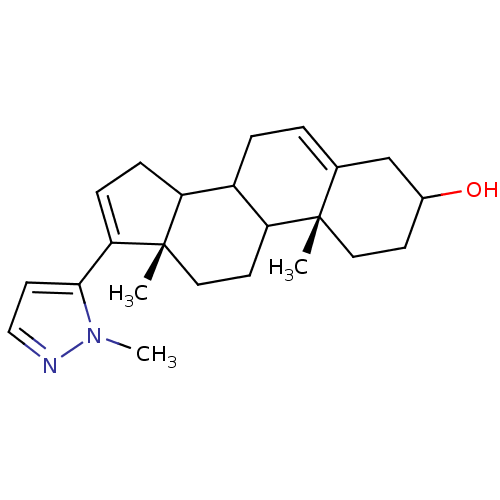
((10R,13S)-10,13-Dimethyl-17-(2-methyl-2H-pyrazol-3...)Show SMILES Cn1nccc1C1=CCC2C3CC=C4CC(O)CC[C@]4(C)C3CC[C@]12C |t:7,13| Show InChI InChI=1S/C23H32N2O/c1-22-11-8-16(26)14-15(22)4-5-17-18-6-7-20(21-10-13-24-25(21)3)23(18,2)12-9-19(17)22/h4,7,10,13,16-19,26H,5-6,8-9,11-12,14H2,1-3H3/t16?,17?,18?,19?,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408270
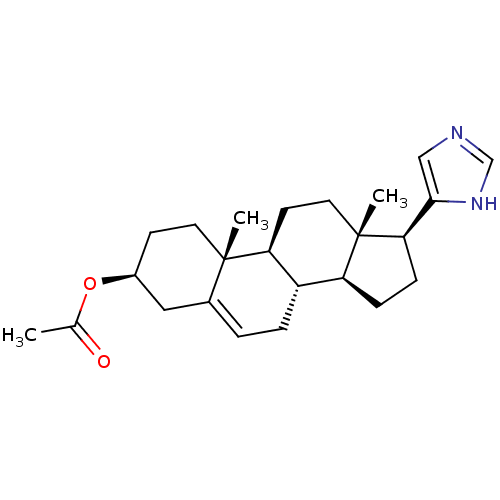
(CHEMBL2112528)Show SMILES CC(=O)O[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC[C@@H]4c4cnc[nH]4)[C@@H]3CC=C2C1 |c:28| Show InChI InChI=1S/C24H34N2O2/c1-15(27)28-17-8-10-23(2)16(12-17)4-5-18-19-6-7-21(22-13-25-14-26-22)24(19,3)11-9-20(18)23/h4,13-14,17-21H,5-12H2,1-3H3,(H,25,26)/t17-,18-,19-,20-,21+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061190
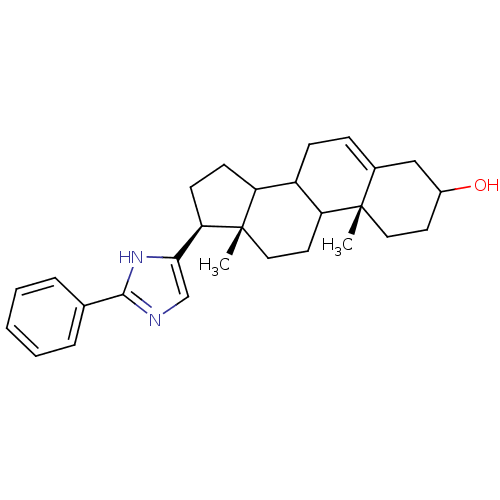
((10R,13S,17S)-10,13-Dimethyl-17-(2-phenyl-1H-imida...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CC[C@@H]2c1cnc([nH]1)-c1ccccc1 |t:7| Show InChI InChI=1S/C28H36N2O/c1-27-14-12-20(31)16-19(27)8-9-21-22-10-11-24(28(22,2)15-13-23(21)27)25-17-29-26(30-25)18-6-4-3-5-7-18/h3-8,17,20-24,31H,9-16H2,1-2H3,(H,29,30)/t20?,21?,22?,23?,24-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061179
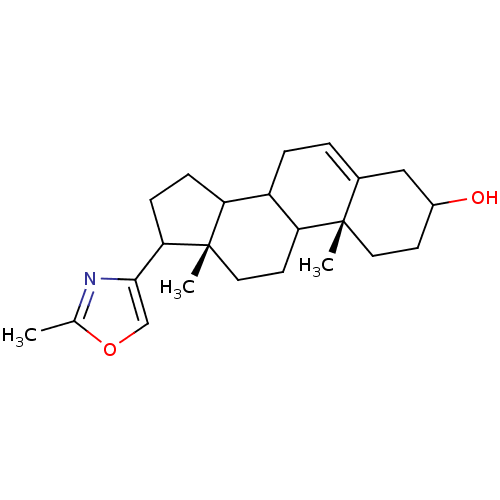
((10R,13S)-10,13-Dimethyl-17-(2-methyl-oxazol-4-yl)...)Show SMILES Cc1nc(co1)C1CCC2C3CC=C4CC(O)CC[C@]4(C)C3CC[C@]12C |t:13| Show InChI InChI=1S/C23H33NO2/c1-14-24-21(13-26-14)20-7-6-18-17-5-4-15-12-16(25)8-10-22(15,2)19(17)9-11-23(18,20)3/h4,13,16-20,25H,5-12H2,1-3H3/t16?,17?,18?,19?,20?,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408266

(CHEMBL2112533)Show SMILES Cc1ncc([nH]1)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |t:13| Show InChI InChI=1S/C23H34N2O/c1-14-24-13-21(25-14)20-7-6-18-17-5-4-15-12-16(26)8-10-22(15,2)19(17)9-11-23(18,20)3/h4,13,16-20,26H,5-12H2,1-3H3,(H,24,25)/t16-,17-,18-,19-,20+,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061187

((10R,13S)-17-(1H-Imidazol-2-yl)-10,13-dimethyl-2,3...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CCC2c1ncc[nH]1 |t:7| Show InChI InChI=1S/C22H32N2O/c1-21-9-7-15(25)13-14(21)3-4-16-17-5-6-19(20-23-11-12-24-20)22(17,2)10-8-18(16)21/h3,11-12,15-19,25H,4-10,13H2,1-2H3,(H,23,24)/t15?,16?,17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061183

((10R,13S,17S)-17-(2H-Imidazol-2-yl)-10,13-dimethyl...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC[C@@H]2C1N=CC=N1 |c:25,27,t:8| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)13-14(21)3-4-16-17-5-6-19(20-23-11-12-24-20)22(17,2)10-8-18(16)21/h11-13,16-20H,3-10H2,1-2H3/t16?,17?,18?,19-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50408274

(CHEMBL2112519)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC1O[C@@]21c1cnc[nH]1 |t:7| Show InChI InChI=1S/C22H30N2O2/c1-20-7-5-14(25)9-13(20)3-4-15-16(20)6-8-21(2)17(15)10-19-22(21,26-19)18-11-23-12-24-18/h3,11-12,14-17,19,25H,4-10H2,1-2H3,(H,23,24)/t14-,15+,16-,17-,19?,20-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061188
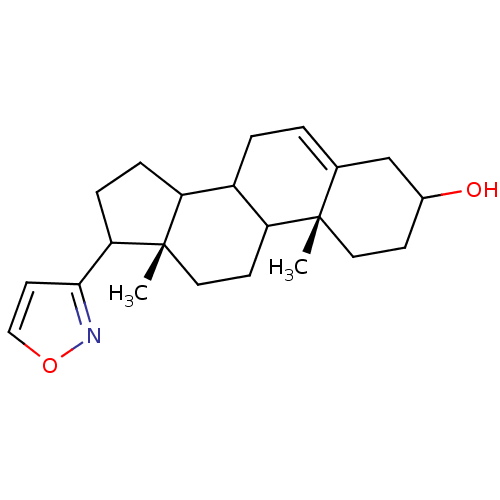
((10R,13S)-17-Isoxazol-3-yl-10,13-dimethyl-2,3,4,7,...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CCC2c1ccon1 |t:7| Show InChI InChI=1S/C22H31NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-25-23-20)22(17,2)11-8-18(16)21/h3,9,12,15-19,24H,4-8,10-11,13H2,1-2H3/t15?,16?,17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50061184
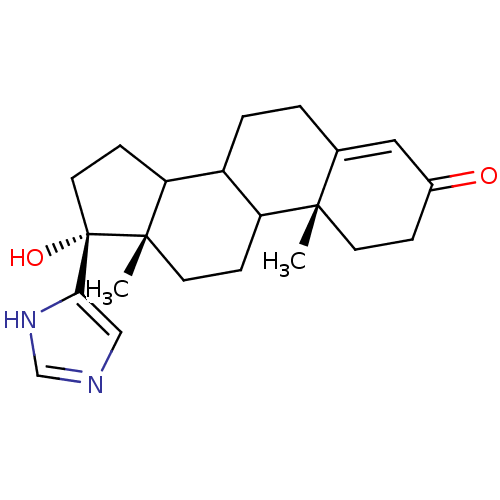
((10R,13S,17R)-17-Hydroxy-17-(1H-imidazol-4-yl)-10,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC[C@]2(O)c1cnc[nH]1 |t:8| Show InChI InChI=1S/C22H30N2O2/c1-20-8-5-15(25)11-14(20)3-4-16-17(20)6-9-21(2)18(16)7-10-22(21,26)19-12-23-13-24-19/h11-13,16-18,26H,3-10H2,1-2H3,(H,23,24)/t16?,17?,18?,20-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408273

(CHEMBL2112522)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2c1ncc[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)13-14(21)3-4-16-17-5-6-19(20-23-11-12-24-20)22(17,2)10-8-18(16)21/h3,6,11-12,15-18,25H,4-5,7-10,13H2,1-2H3,(H,23,24)/t15-,16-,17-,18-,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408265

(CHEMBL2112534)Show SMILES CC(=O)O[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC=C4c4cnc[nH]4)[C@@H]3CC=C2C1 |c:16,28| Show InChI InChI=1S/C24H32N2O2/c1-15(27)28-17-8-10-23(2)16(12-17)4-5-18-19-6-7-21(22-13-25-14-26-22)24(19,3)11-9-20(18)23/h4,7,13-14,17-20H,5-6,8-12H2,1-3H3,(H,25,26)/t17-,18-,19-,20-,23-,24-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061166

((10R,13S)-10,13-Dimethyl-17-(2-methyl-2H-pyrazol-3...)Show SMILES Cn1nccc1C1=CCC2C3CC=C4CC(O)CC[C@]4(C)C3CC[C@]12C |t:7,13| Show InChI InChI=1S/C23H32N2O/c1-22-11-8-16(26)14-15(22)4-5-17-18-6-7-20(21-10-13-24-25(21)3)23(18,2)12-9-19(17)22/h4,7,10,13,16-19,26H,5-6,8-9,11-12,14H2,1-3H3/t16?,17?,18?,19?,22-,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061173

((10R,13S)-10,13-Dimethyl-17-(2-phenyl-2H-pyrazol-3...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CC=C2c1ccnn1-c1ccccc1 |c:21,t:7| Show InChI InChI=1S/C28H34N2O/c1-27-15-12-21(31)18-19(27)8-9-22-23-10-11-25(28(23,2)16-13-24(22)27)26-14-17-29-30(26)20-6-4-3-5-7-20/h3-8,11,14,17,21-24,31H,9-10,12-13,15-16,18H2,1-2H3/t21?,22?,23?,24?,27-,28-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061179

((10R,13S)-10,13-Dimethyl-17-(2-methyl-oxazol-4-yl)...)Show SMILES Cc1nc(co1)C1CCC2C3CC=C4CC(O)CC[C@]4(C)C3CC[C@]12C |t:13| Show InChI InChI=1S/C23H33NO2/c1-14-24-21(13-26-14)20-7-6-18-17-5-4-15-12-16(25)8-10-22(15,2)19(17)9-11-23(18,20)3/h4,13,16-20,25H,5-12H2,1-3H3/t16?,17?,18?,19?,20?,22-,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061186

((10R,13S)-17-Isoxazol-5-yl-10,13-dimethyl-2,3,4,7,...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CCC2c1ccno1 |t:7| Show InChI InChI=1S/C22H31NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-23-25-20)22(17,2)11-8-18(16)21/h3,9,12,15-19,24H,4-8,10-11,13H2,1-2H3/t15?,16?,17?,18?,19?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061188

((10R,13S)-17-Isoxazol-3-yl-10,13-dimethyl-2,3,4,7,...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CCC2c1ccon1 |t:7| Show InChI InChI=1S/C22H31NO2/c1-21-10-7-15(24)13-14(21)3-4-16-17-5-6-19(20-9-12-25-23-20)22(17,2)11-8-18(16)21/h3,9,12,15-19,24H,4-8,10-11,13H2,1-2H3/t15?,16?,17?,18?,19?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061189

((10R,13S)-10,13-Dimethyl-17-(2H-pyrazol-3-yl)-2,3,...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CCC2c1ccn[nH]1 |t:7| Show InChI InChI=1S/C22H32N2O/c1-21-10-7-15(25)13-14(21)3-4-16-17-5-6-19(20-9-12-23-24-20)22(17,2)11-8-18(16)21/h3,9,12,15-19,25H,4-8,10-11,13H2,1-2H3,(H,23,24)/t15?,16?,17?,18?,19?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061181

((10R,13S)-10,13-Dimethyl-17-(1-methyl-1H-pyrazol-3...)Show SMILES Cn1ccc(n1)C1=CCC2C3CC=C4CC(O)CC[C@]4(C)C3CC[C@]12C |t:7,13| Show InChI InChI=1S/C23H32N2O/c1-22-11-8-16(26)14-15(22)4-5-17-18-6-7-20(21-10-13-25(3)24-21)23(18,2)12-9-19(17)22/h4,7,10,13,16-19,26H,5-6,8-9,11-12,14H2,1-3H3/t16?,17?,18?,19?,22-,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061183

((10R,13S,17S)-17-(2H-Imidazol-2-yl)-10,13-dimethyl...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC[C@@H]2C1N=CC=N1 |c:25,27,t:8| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)13-14(21)3-4-16-17-5-6-19(20-23-11-12-24-20)22(17,2)10-8-18(16)21/h11-13,16-20H,3-10H2,1-2H3/t16?,17?,18?,19-,21+,22+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061190

((10R,13S,17S)-10,13-Dimethyl-17-(2-phenyl-1H-imida...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CC[C@@H]2c1cnc([nH]1)-c1ccccc1 |t:7| Show InChI InChI=1S/C28H36N2O/c1-27-14-12-20(31)16-19(27)8-9-21-22-10-11-24(28(22,2)15-13-23(21)27)25-17-29-26(30-25)18-6-4-3-5-7-18/h3-8,17,20-24,31H,9-16H2,1-2H3,(H,29,30)/t20?,21?,22?,23?,24-,27+,28+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50061187

((10R,13S)-17-(1H-Imidazol-2-yl)-10,13-dimethyl-2,3...)Show SMILES C[C@]12CCC3C(CC=C4CC(O)CC[C@]34C)C1CCC2c1ncc[nH]1 |t:7| Show InChI InChI=1S/C22H32N2O/c1-21-9-7-15(25)13-14(21)3-4-16-17-5-6-19(20-23-11-12-24-20)22(17,2)10-8-18(16)21/h3,11-12,15-19,25H,4-10,13H2,1-2H3,(H,23,24)/t15?,16?,17?,18?,19?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408270

(CHEMBL2112528)Show SMILES CC(=O)O[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC[C@@H]4c4cnc[nH]4)[C@@H]3CC=C2C1 |c:28| Show InChI InChI=1S/C24H34N2O2/c1-15(27)28-17-8-10-23(2)16(12-17)4-5-18-19-6-7-21(22-13-25-14-26-22)24(19,3)11-9-20(18)23/h4,13-14,17-21H,5-12H2,1-3H3,(H,25,26)/t17-,18-,19-,20-,21+,23-,24-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicual micrososmal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408266

(CHEMBL2112533)Show SMILES Cc1ncc([nH]1)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |t:13| Show InChI InChI=1S/C23H34N2O/c1-14-24-13-21(25-14)20-7-6-18-17-5-4-15-12-16(26)8-10-22(15,2)19(17)9-11-23(18,20)3/h4,13,16-20,26H,5-12H2,1-3H3,(H,24,25)/t16-,17-,18-,19-,20+,22-,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50408268

(CHEMBL2112524)Show SMILES CC(=O)O[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC[C@@H]4C4=NC(NC4)C(C)=O)[C@@H]3CC=C2C1 |c:31,t:19| Show InChI InChI=1S/C26H38N2O3/c1-15(29)24-27-14-23(28-24)22-8-7-20-19-6-5-17-13-18(31-16(2)30)9-11-25(17,3)21(19)10-12-26(20,22)4/h5,18-22,24,27H,6-14H2,1-4H3/t18-,19-,20-,21-,22+,24?,25-,26-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat testicular microsomal Cytochrome P450 17A1 |
J Med Chem 40: 3297-304 (1997)
Article DOI: 10.1021/jm970337k
BindingDB Entry DOI: 10.7270/Q2ZS2VMD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data