

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM427470 (US10538497, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509644 (CHEMBL4538516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509629 (CHEMBL4436560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509636 (CHEMBL4441030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509633 (CHEMBL4521926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509638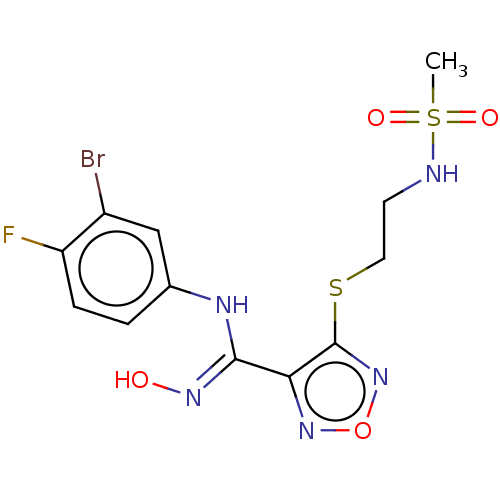 (CHEMBL4533342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509623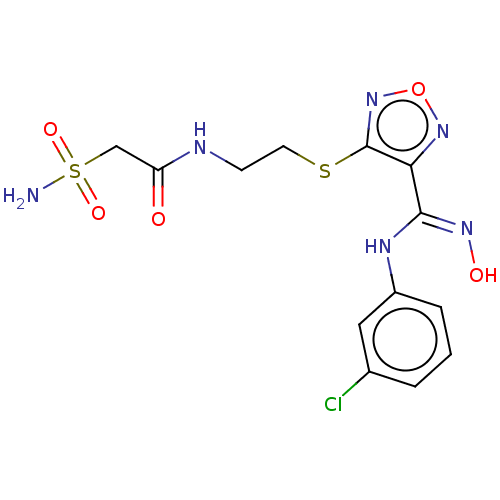 (CHEMBL4585089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509628 (CHEMBL4434929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509630 (CHEMBL4448442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509637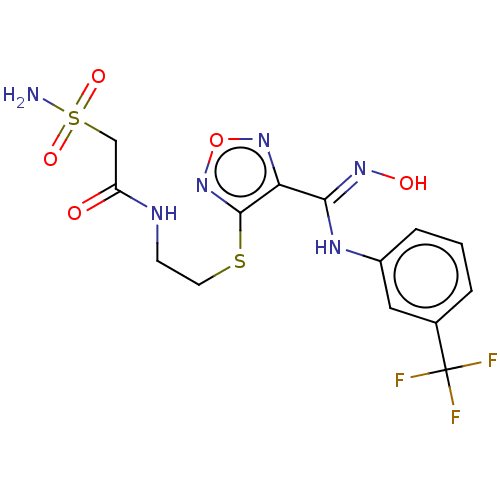 (CHEMBL4449948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM430022 (US10538497, Example 212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509641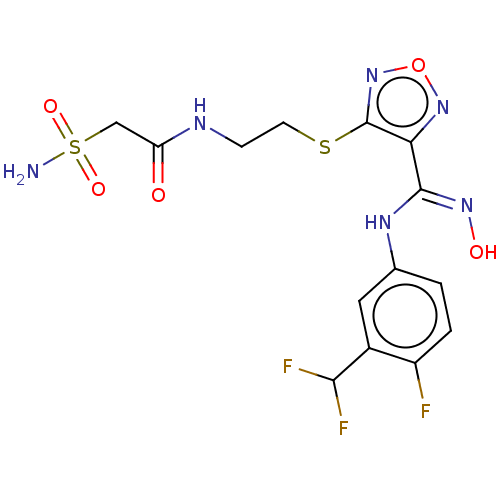 (CHEMBL4455438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509639 (CHEMBL4439896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509634 (CHEMBL4437616) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50235921 (CHEMBL584991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509643 (CHEMBL4578545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509632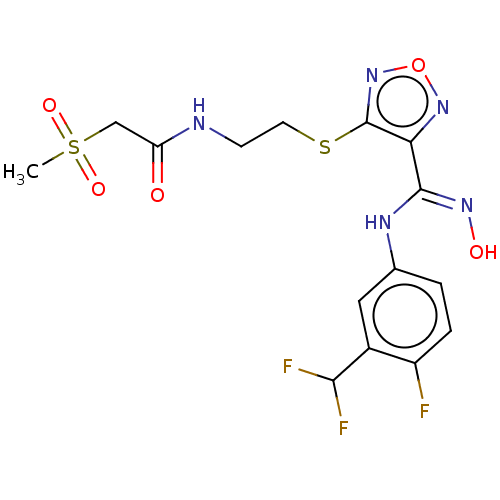 (CHEMBL4441246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509625 (CHEMBL4573030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509624 (CHEMBL4533432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509627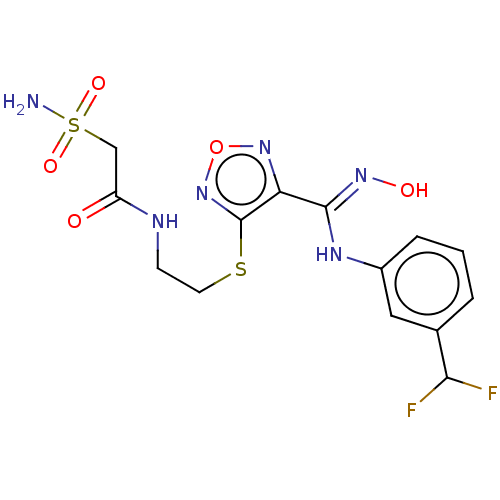 (CHEMBL4475499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509631 (CHEMBL4451194) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509623 (CHEMBL4585089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509626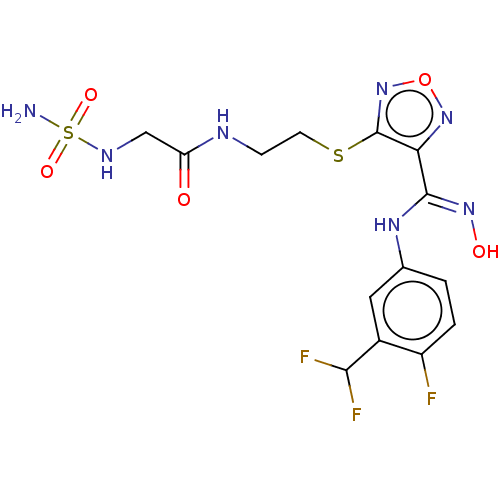 (CHEMBL4549976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509636 (CHEMBL4441030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509640 (CHEMBL4545974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509635 (CHEMBL4465837) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509642 (CHEMBL4461796) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509643 (CHEMBL4578545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509634 (CHEMBL4437616) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509630 (CHEMBL4448442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509641 (CHEMBL4455438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509639 (CHEMBL4439896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509631 (CHEMBL4451194) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509626 (CHEMBL4549976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509632 (CHEMBL4441246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509628 (CHEMBL4434929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM427470 (US10538497, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509627 (CHEMBL4475499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509629 (CHEMBL4436560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509633 (CHEMBL4521926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509637 (CHEMBL4449948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509635 (CHEMBL4465837) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM430022 (US10538497, Example 212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509644 (CHEMBL4538516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50235921 (CHEMBL584991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509623 (CHEMBL4585089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition IDO1 in LPS/IFN-gamma stimulated human whole blood assessed as reduction in tryptophan/kynurenine level preincubated for 15 mins followed ... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509640 (CHEMBL4545974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50509642 (CHEMBL4461796) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... | ACS Med Chem Lett 11: 179-187 (2020) Article DOI: 10.1021/acsmedchemlett.9b00572 BindingDB Entry DOI: 10.7270/Q2GX4FWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |