

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged BRD2 BD2 domain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by AlphaScreen assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged BRD4 BD1 domain using H-SGRGK(Ac)GGK(Ac)GLGK-(Ac)GGAK(Ac)RHRK(Biotin)-OH as substrate preincubated with en... | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged BRD3 BD2 domain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by AlphaScreen assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50511231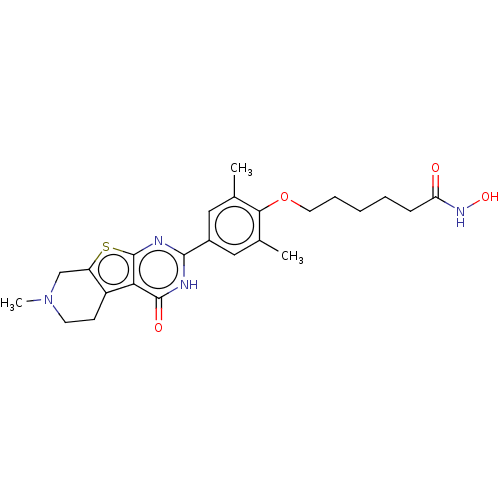 (CHEMBL4514808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511231 (CHEMBL4514808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged BRD3 BD1 domain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by AlphaScreen assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged BRD4 BD2 domain using H-SGRGK(Ac)GGK(Ac)GLGK-(Ac)GGAK(Ac)RHRK(Biotin)-OH as substrate preincubated with en... | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511231 (CHEMBL4514808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50511231 (CHEMBL4514808) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511247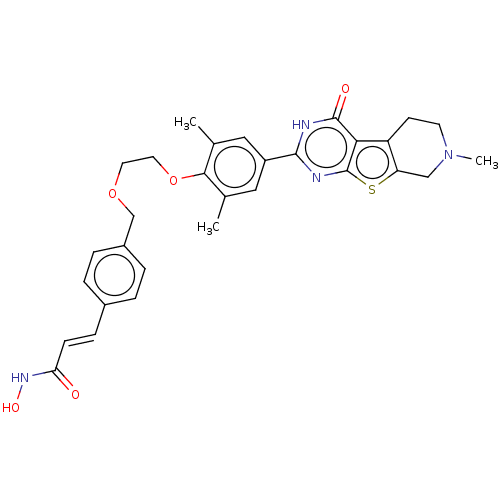 (CHEMBL4465878) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511228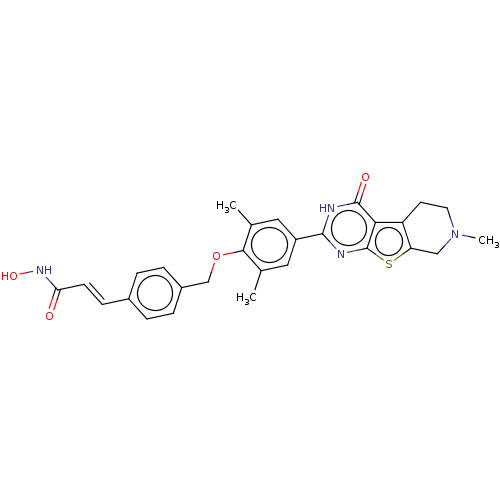 (CHEMBL4548155) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511229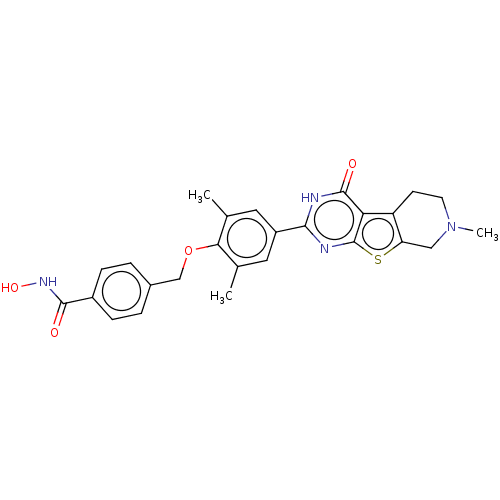 (CHEMBL4592260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511230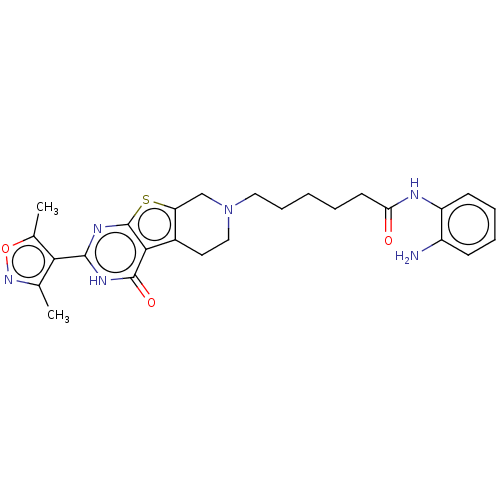 (CHEMBL4438753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511244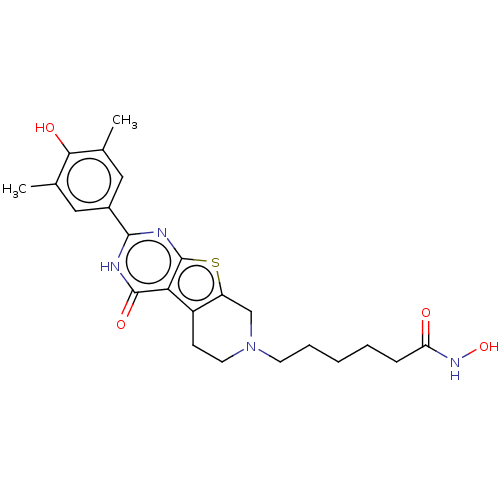 (CHEMBL4548542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged BRDT BD1 domain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by AlphaScreen assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511236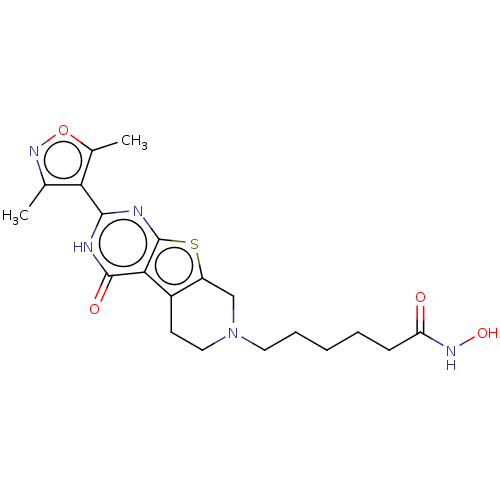 (CHEMBL4530039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511244 (CHEMBL4548542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511233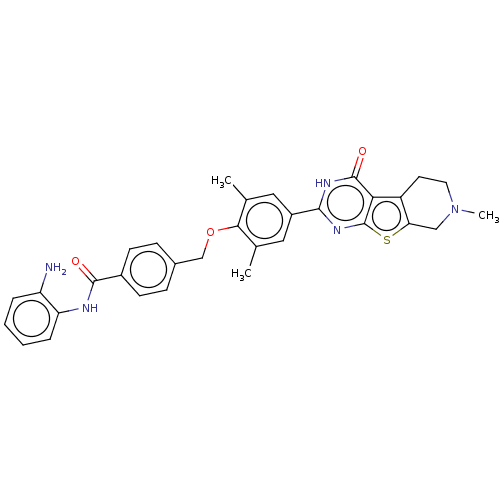 (CHEMBL4471956) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged BRD2 BD1 domain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by AlphaScreen assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511236 (CHEMBL4530039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511247 (CHEMBL4465878) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511234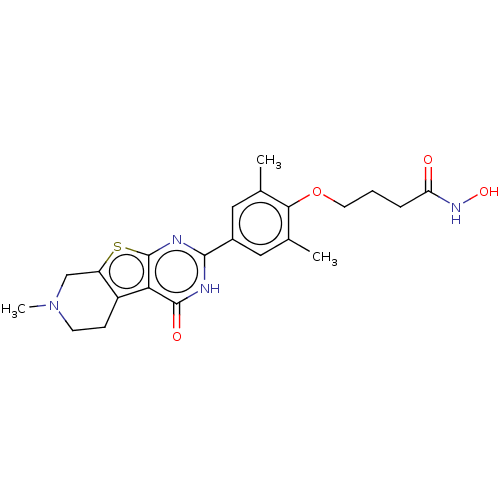 (CHEMBL4551888) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50511231 (CHEMBL4514808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511245 (CHEMBL4555072) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511229 (CHEMBL4592260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511230 (CHEMBL4438753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511228 (CHEMBL4548155) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511234 (CHEMBL4551888) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511246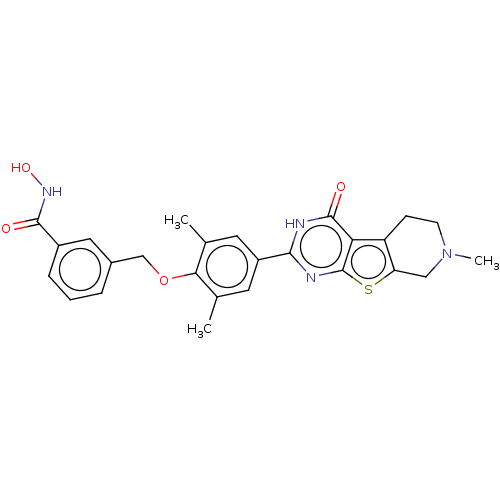 (CHEMBL4443980) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511254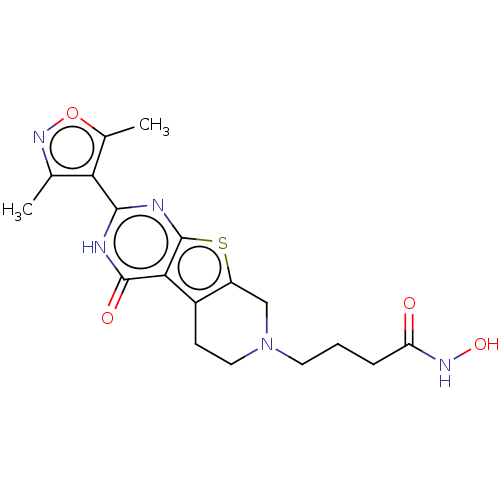 (CHEMBL4436262) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511248 (CHEMBL4581033) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511248 (CHEMBL4581033) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 391 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511235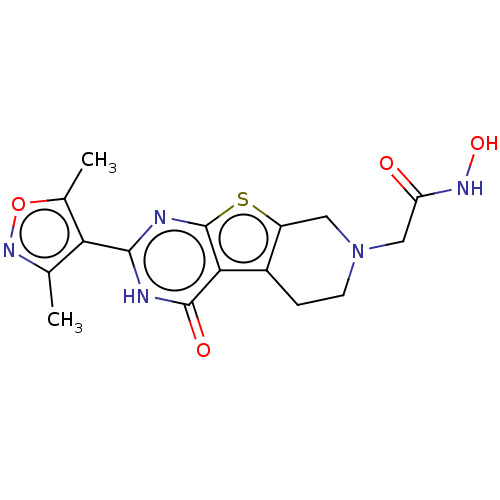 (CHEMBL4553337) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511254 (CHEMBL4436262) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 464 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511235 (CHEMBL4553337) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 489 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511231 (CHEMBL4514808) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of GST-tagged BRD4 bromodomain (unknown origin) using biotinylated histone H4KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50511246 (CHEMBL4443980) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50251045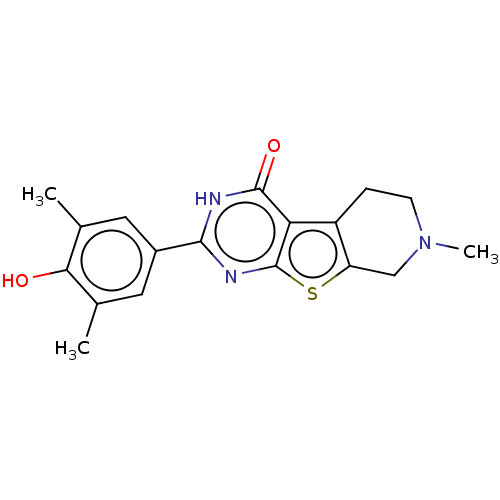 (CHEMBL4077274) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of GST-tagged BRD4 bromodomain (unknown origin) using biotinylated histone H4KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50511232 (CHEMBL4547886) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50511231 (CHEMBL4514808) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 923 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) using biotinylated histone H3 KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511247 (CHEMBL4465878) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of GST-tagged BRD4 bromodomain (unknown origin) using biotinylated histone H4KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511231 (CHEMBL4514808) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged BRD4 BD1 domain using H-SGRGK(Ac)GGK(Ac)GLGK-(Ac)GGAK(Ac)RHRK(Biotin)-OH as substrate preincubated with en... | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511234 (CHEMBL4551888) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of GST-tagged BRD4 bromodomain (unknown origin) using biotinylated histone H4KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511228 (CHEMBL4548155) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center of Biotherapy Curated by ChEMBL | Assay Description Inhibition of GST-tagged BRD4 bromodomain (unknown origin) using biotinylated histone H4KAc peptide (1 to 21 residues) as substrate by HTRF assay | J Med Chem 63: 3678-3700 (2020) Article DOI: 10.1021/acs.jmedchem.9b02178 BindingDB Entry DOI: 10.7270/Q27W6GHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 118 total ) | Next | Last >> |