 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50008514
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50008514 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520035
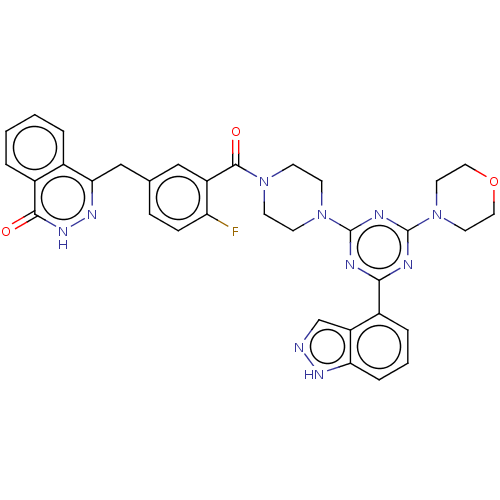
(CHEMBL4461392)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C34H31FN10O3/c35-27-9-8-21(19-29-22-4-1-2-5-24(22)31(46)42-41-29)18-25(27)32(47)43-10-12-44(13-11-43)33-37-30(23-6-3-7-28-26(23)20-36-40-28)38-34(39-33)45-14-16-48-17-15-45/h1-9,18,20H,10-17,19H2,(H,36,40)(H,42,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520036
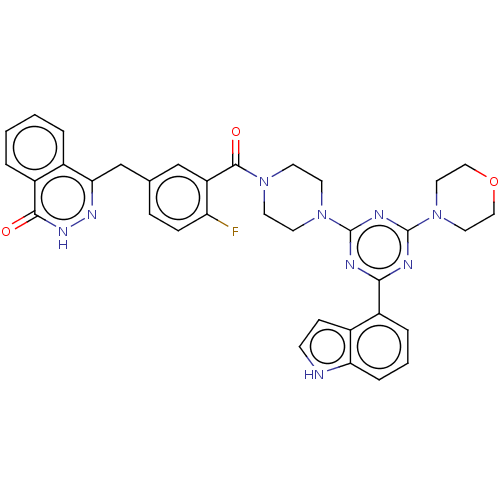
(CHEMBL4437593)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C35H32FN9O3/c36-28-9-8-22(21-30-23-4-1-2-5-26(23)32(46)42-41-30)20-27(28)33(47)43-12-14-44(15-13-43)34-38-31(25-6-3-7-29-24(25)10-11-37-29)39-35(40-34)45-16-18-48-19-17-45/h1-11,20,37H,12-19,21H2,(H,42,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520043

(CHEMBL4549595)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1ccc2cn[nH]c2c1)N1CCOCC1 Show InChI InChI=1S/C34H31FN10O3/c35-27-8-5-21(18-29-24-3-1-2-4-25(24)31(46)42-41-29)17-26(27)32(47)43-9-11-44(12-10-43)33-37-30(22-6-7-23-20-36-40-28(23)19-22)38-34(39-33)45-13-15-48-16-14-45/h1-8,17,19-20H,9-16,18H2,(H,36,40)(H,42,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520026

(CHEMBL4525998)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1ccc2[nH]ccc2c1)N1CCOCC1 Show InChI InChI=1S/C35H32FN9O3/c36-28-7-5-22(20-30-25-3-1-2-4-26(25)32(46)42-41-30)19-27(28)33(47)43-11-13-44(14-12-43)34-38-31(24-6-8-29-23(21-24)9-10-37-29)39-35(40-34)45-15-17-48-18-16-45/h1-10,19,21,37H,11-18,20H2,(H,42,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520031
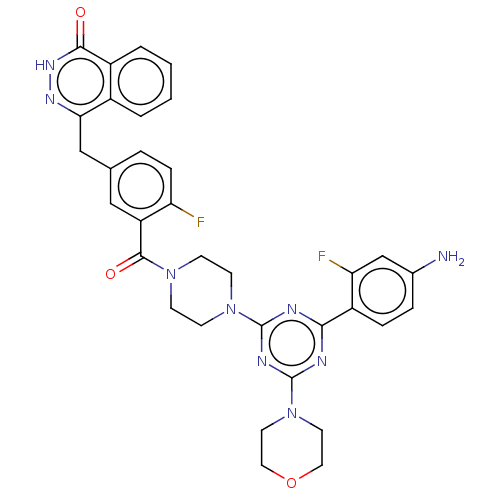
(CHEMBL4436980)Show SMILES Nc1ccc(c(F)c1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H31F2N9O3/c34-26-8-5-20(18-28-22-3-1-2-4-23(22)30(45)41-40-28)17-25(26)31(46)42-9-11-43(12-10-42)32-37-29(24-7-6-21(36)19-27(24)35)38-33(39-32)44-13-15-47-16-14-44/h1-8,17,19H,9-16,18,36H2,(H,41,45) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520042

(CHEMBL4571423)Show SMILES Nc1ccc(cc1F)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H31F2N9O3/c34-25-7-5-20(18-28-22-3-1-2-4-23(22)30(45)41-40-28)17-24(25)31(46)42-9-11-43(12-10-42)32-37-29(21-6-8-27(36)26(35)19-21)38-33(39-32)44-13-15-47-16-14-44/h1-8,17,19H,9-16,18,36H2,(H,41,45) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520033
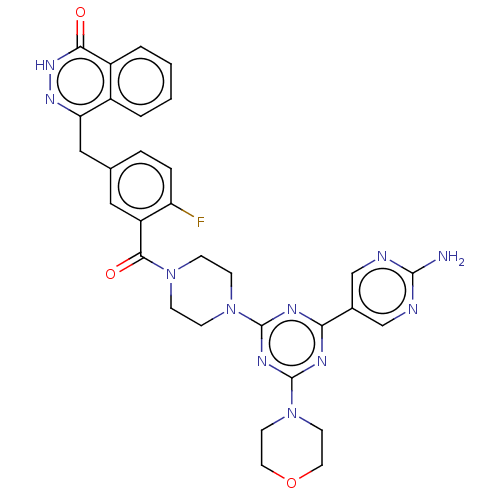
(CHEMBL4461382)Show SMILES Nc1ncc(cn1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C31H30FN11O3/c32-24-6-5-19(16-25-21-3-1-2-4-22(21)27(44)40-39-25)15-23(24)28(45)41-7-9-42(10-8-41)30-36-26(20-17-34-29(33)35-18-20)37-31(38-30)43-11-13-46-14-12-43/h1-6,15,17-18H,7-14,16H2,(H,40,44)(H2,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP2 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520032

(CHEMBL4551779)Show SMILES Nc1ccc(cn1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C32H31FN10O3/c33-25-7-5-20(18-26-22-3-1-2-4-23(22)29(44)40-39-26)17-24(25)30(45)41-9-11-42(12-10-41)31-36-28(21-6-8-27(34)35-19-21)37-32(38-31)43-13-15-46-16-14-43/h1-8,17,19H,9-16,18H2,(H2,34,35)(H,40,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520030
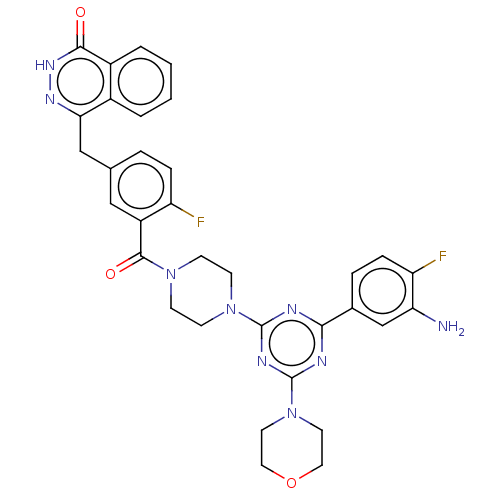
(CHEMBL4436366)Show SMILES Nc1cc(ccc1F)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H31F2N9O3/c34-25-7-5-20(18-28-22-3-1-2-4-23(22)30(45)41-40-28)17-24(25)31(46)42-9-11-43(12-10-42)32-37-29(21-6-8-26(35)27(36)19-21)38-33(39-32)44-13-15-47-16-14-44/h1-8,17,19H,9-16,18,36H2,(H,41,45) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520041

(CHEMBL4446789)Show SMILES Nc1ncc(cc1C(F)(F)F)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H30F4N10O3/c34-25-6-5-19(16-26-21-3-1-2-4-22(21)29(48)44-43-26)15-23(25)30(49)45-7-9-46(10-8-45)31-40-28(41-32(42-31)47-11-13-50-14-12-47)20-17-24(33(35,36)37)27(38)39-18-20/h1-6,15,17-18H,7-14,16H2,(H2,38,39)(H,44,48) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520027
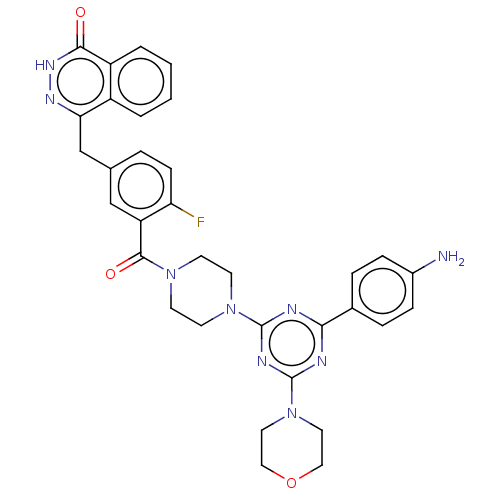
(CHEMBL4438927)Show SMILES Nc1ccc(cc1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H32FN9O3/c34-27-10-5-21(20-28-24-3-1-2-4-25(24)30(44)40-39-28)19-26(27)31(45)41-11-13-42(14-12-41)32-36-29(22-6-8-23(35)9-7-22)37-33(38-32)43-15-17-46-18-16-43/h1-10,19H,11-18,20,35H2,(H,40,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520040

(CHEMBL4438741)Show SMILES Nc1ncc(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C31H28FN9O3/c32-24-6-5-18(14-25-20-3-1-2-4-21(20)29(42)39-38-25)13-23(24)30(43)41-8-7-22-26(17-41)36-27(19-15-34-31(33)35-16-19)37-28(22)40-9-11-44-12-10-40/h1-6,13,15-16H,7-12,14,17H2,(H,39,42)(H2,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP2 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520040

(CHEMBL4438741)Show SMILES Nc1ncc(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C31H28FN9O3/c32-24-6-5-18(14-25-20-3-1-2-4-21(20)29(42)39-38-25)13-23(24)30(43)41-8-7-22-26(17-41)36-27(19-15-34-31(33)35-16-19)37-28(22)40-9-11-44-12-10-40/h1-6,13,15-16H,7-12,14,17H2,(H,39,42)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520029
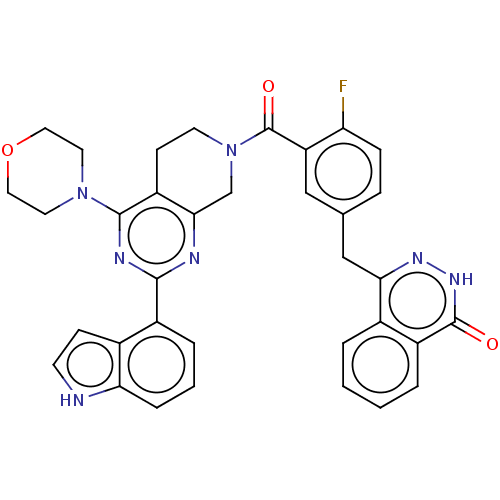
(CHEMBL4593274)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCc2c(C1)nc(nc2N1CCOCC1)-c1cccc2[nH]ccc12 Show InChI InChI=1S/C35H30FN7O3/c36-28-9-8-21(19-30-22-4-1-2-5-25(22)34(44)41-40-30)18-27(28)35(45)43-13-11-26-31(20-43)38-32(39-33(26)42-14-16-46-17-15-42)24-6-3-7-29-23(24)10-12-37-29/h1-10,12,18,37H,11,13-17,19-20H2,(H,41,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520039

(CHEMBL4531016)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCc2c(C1)nc(nc2N1CCOCC1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C34H29FN8O3/c35-27-9-8-20(17-29-21-4-1-2-5-23(21)33(44)41-40-29)16-25(27)34(45)43-11-10-24-30(19-43)37-31(38-32(24)42-12-14-46-15-13-42)22-6-3-7-28-26(22)18-36-39-28/h1-9,16,18H,10-15,17,19H2,(H,36,39)(H,41,44) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 2 hrs by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520028

(CHEMBL4551630)Show SMILES Nc1ccc(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C32H29FN8O3/c33-25-7-5-19(16-26-21-3-1-2-4-22(21)31(42)39-38-26)15-24(25)32(43)41-10-9-23-27(18-41)36-29(20-6-8-28(34)35-17-20)37-30(23)40-11-13-44-14-12-40/h1-8,15,17H,9-14,16,18H2,(H2,34,35)(H,39,42) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520034

(CHEMBL4541731)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C33H30F4N10O3/c34-25-6-5-19(16-26-20-3-1-2-4-21(20)29(48)44-43-26)15-22(25)30(49)45-7-9-46(10-8-45)31-40-28(41-32(42-31)47-11-13-50-14-12-47)23-18-39-27(38)17-24(23)33(35,36)37/h1-6,15,17-18H,7-14,16H2,(H2,38,39)(H,44,48) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50520038

(CHEMBL4465869)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C34H31F4N9O3/c35-26-6-5-20(16-28-21-3-1-2-4-22(21)31(48)44-43-28)15-23(26)32(49)46-9-7-45(8-10-46)30-18-27(41-33(42-30)47-11-13-50-14-12-47)24-19-40-29(39)17-25(24)34(36,37)38/h1-6,15,17-19H,7-14,16H2,(H2,39,40)(H,44,48) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PARP1 using histone as substrate after 1 hr in presence of biotinylated NAD+ by ELISA |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520028

(CHEMBL4551630)Show SMILES Nc1ccc(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F Show InChI InChI=1S/C32H29FN8O3/c33-25-7-5-19(16-26-21-3-1-2-4-22(21)31(42)39-38-26)15-24(25)32(43)41-10-9-23-27(18-41)36-29(20-6-8-28(34)35-17-20)37-30(23)40-11-13-44-14-12-40/h1-8,15,17H,9-14,16,18H2,(H2,34,35)(H,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520029

(CHEMBL4593274)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCc2c(C1)nc(nc2N1CCOCC1)-c1cccc2[nH]ccc12 Show InChI InChI=1S/C35H30FN7O3/c36-28-9-8-21(19-30-22-4-1-2-5-25(22)34(44)41-40-30)18-27(28)35(45)43-13-11-26-31(20-43)38-32(39-33(26)42-14-16-46-17-15-42)24-6-3-7-29-23(24)10-12-37-29/h1-10,12,18,37H,11,13-17,19-20H2,(H,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520039

(CHEMBL4531016)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCc2c(C1)nc(nc2N1CCOCC1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C34H29FN8O3/c35-27-9-8-20(17-29-21-4-1-2-5-23(21)33(44)41-40-29)16-25(27)34(45)43-11-10-24-30(19-43)37-31(38-32(24)42-12-14-46-15-13-42)22-6-3-7-28-26(22)18-36-39-28/h1-9,16,18H,10-15,17,19H2,(H,36,39)(H,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380363
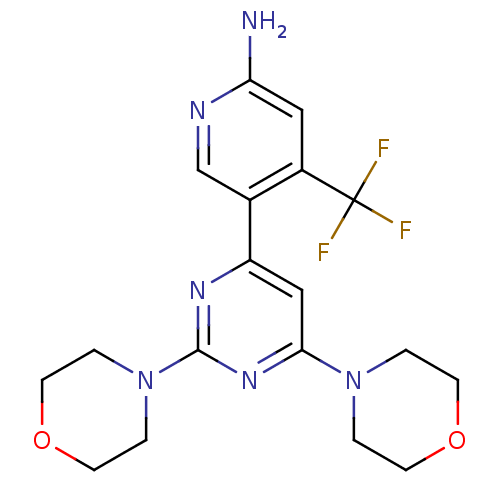
(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520033

(CHEMBL4461382)Show SMILES Nc1ncc(cn1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C31H30FN11O3/c32-24-6-5-19(16-25-21-3-1-2-4-22(21)27(44)40-39-25)15-23(24)28(45)41-7-9-42(10-8-41)30-36-26(20-17-34-29(33)35-18-20)37-31(38-30)43-11-13-46-14-12-43/h1-6,15,17-18H,7-14,16H2,(H,40,44)(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 2 hrs by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520032

(CHEMBL4551779)Show SMILES Nc1ccc(cn1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C32H31FN10O3/c33-25-7-5-20(18-26-22-3-1-2-4-23(22)29(44)40-39-26)17-24(25)30(45)41-9-11-42(12-10-41)31-36-28(21-6-8-27(34)35-19-21)37-32(38-31)43-13-15-46-16-14-43/h1-8,17,19H,9-16,18H2,(H2,34,35)(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520034

(CHEMBL4541731)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C33H30F4N10O3/c34-25-6-5-19(16-26-20-3-1-2-4-21(20)29(48)44-43-26)15-22(25)30(49)45-7-9-46(10-8-45)31-40-28(41-32(42-31)47-11-13-50-14-12-47)23-18-39-27(38)17-24(23)33(35,36)37/h1-6,15,17-18H,7-14,16H2,(H2,38,39)(H,44,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520038

(CHEMBL4465869)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C34H31F4N9O3/c35-26-6-5-20(16-28-21-3-1-2-4-22(21)31(48)44-43-28)15-23(26)32(49)46-9-7-45(8-10-46)30-18-27(41-33(42-30)47-11-13-50-14-12-47)24-19-40-29(39)17-25(24)34(36,37)38/h1-6,15,17-19H,7-14,16H2,(H2,39,40)(H,44,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520027

(CHEMBL4438927)Show SMILES Nc1ccc(cc1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H32FN9O3/c34-27-10-5-21(20-28-24-3-1-2-4-25(24)30(44)40-39-28)19-26(27)31(45)41-11-13-42(14-12-41)32-36-29(22-6-8-23(35)9-7-22)37-33(38-32)43-15-17-46-18-16-43/h1-10,19H,11-18,20,35H2,(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520035

(CHEMBL4461392)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C34H31FN10O3/c35-27-9-8-21(19-29-22-4-1-2-5-24(22)31(46)42-41-29)18-25(27)32(47)43-10-12-44(13-11-43)33-37-30(23-6-3-7-28-26(23)20-36-40-28)38-34(39-33)45-14-16-48-17-15-45/h1-9,18,20H,10-17,19H2,(H,36,40)(H,42,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520042

(CHEMBL4571423)Show SMILES Nc1ccc(cc1F)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H31F2N9O3/c34-25-7-5-20(18-28-22-3-1-2-4-23(22)30(45)41-40-28)17-24(25)31(46)42-9-11-43(12-10-42)32-37-29(21-6-8-27(36)26(35)19-21)38-33(39-32)44-13-15-47-16-14-44/h1-8,17,19H,9-16,18,36H2,(H,41,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 562 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520036

(CHEMBL4437593)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C35H32FN9O3/c36-28-9-8-22(21-30-23-4-1-2-5-26(23)32(46)42-41-30)20-27(28)33(47)43-12-14-44(15-13-43)34-38-31(25-6-3-7-29-24(25)10-11-37-29)39-35(40-34)45-16-18-48-19-17-45/h1-11,20,37H,12-19,21H2,(H,42,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520026

(CHEMBL4525998)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1ccc2[nH]ccc2c1)N1CCOCC1 Show InChI InChI=1S/C35H32FN9O3/c36-28-7-5-22(20-30-25-3-1-2-4-26(25)32(46)42-41-30)19-27(28)33(47)43-11-13-44(14-12-43)34-38-31(24-6-8-29-23(21-24)9-10-37-29)39-35(40-34)45-15-17-48-18-16-45/h1-10,19,21,37H,11-18,20H2,(H,42,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 692 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520031

(CHEMBL4436980)Show SMILES Nc1ccc(c(F)c1)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H31F2N9O3/c34-26-8-5-20(18-28-22-3-1-2-4-23(22)30(45)41-40-28)17-25(26)31(46)42-9-11-43(12-10-42)32-37-29(24-7-6-21(36)19-27(24)35)38-33(39-32)44-13-15-47-16-14-44/h1-8,17,19H,9-16,18,36H2,(H,41,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50520037

(CHEMBL4470724)Show SMILES Nc1cc(c(cn1)-c1nc2CN(CCc2c(n1)N1CCOCC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(F)(F)F Show InChI InChI=1S/C33H28F4N8O3/c34-25-6-5-18(14-26-19-3-1-2-4-20(19)31(46)43-42-26)13-22(25)32(47)45-8-7-21-27(17-45)40-29(41-30(21)44-9-11-48-12-10-44)23-16-39-28(38)15-24(23)33(35,36)37/h1-6,13,15-16H,7-12,14,17H2,(H2,38,39)(H,43,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 832 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520041

(CHEMBL4446789)Show SMILES Nc1ncc(cc1C(F)(F)F)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H30F4N10O3/c34-25-6-5-19(16-26-21-3-1-2-4-22(21)29(48)44-43-26)15-23(25)30(49)45-7-9-46(10-8-45)31-40-28(41-32(42-31)47-11-13-50-14-12-47)20-17-24(33(35,36)37)27(38)39-18-20/h1-6,15,17-18H,7-14,16H2,(H2,38,39)(H,44,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520030

(CHEMBL4436366)Show SMILES Nc1cc(ccc1F)-c1nc(nc(n1)N1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)N1CCOCC1 Show InChI InChI=1S/C33H31F2N9O3/c34-25-7-5-20(18-28-22-3-1-2-4-23(22)30(45)41-40-28)17-24(25)31(46)42-9-11-43(12-10-42)32-37-29(21-6-8-26(35)27(36)19-21)38-33(39-32)44-13-15-47-16-14-44/h1-8,17,19H,9-16,18,36H2,(H,41,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50520043

(CHEMBL4549595)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)c1nc(nc(n1)-c1ccc2cn[nH]c2c1)N1CCOCC1 Show InChI InChI=1S/C34H31FN10O3/c35-27-8-5-21(18-29-24-3-1-2-4-25(24)31(46)42-41-29)17-26(27)32(47)43-9-11-44(12-10-43)33-37-30(22-6-7-23-20-36-40-28(23)19-22)38-34(39-33)45-13-15-48-16-14-45/h1-8,17,19-20H,9-16,18H2,(H,36,40)(H,42,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by ADP-glo plus luminescence assay |
J Med Chem 63: 122-139 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00622
BindingDB Entry DOI: 10.7270/Q21G0QPD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data