

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50098378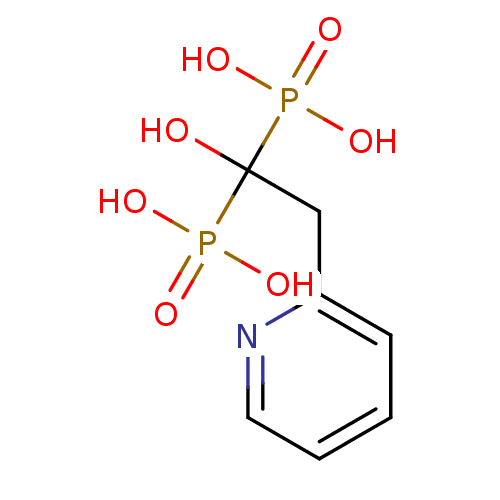 ((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50443054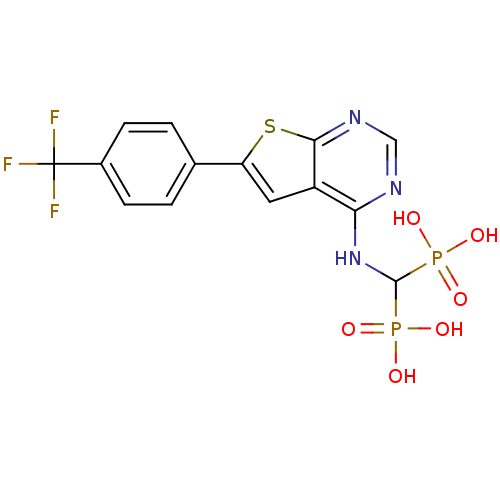 (CHEMBL3087934 | US11279719, Example C-12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520638 (CHEMBL4471037 | US11279719, Example I-18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520641 (CHEMBL4455060 | US11279719, Example I-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520640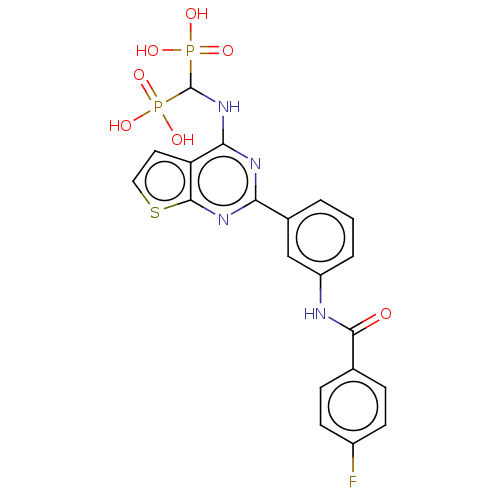 (CHEMBL4472025 | US11279719, Example I-34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520639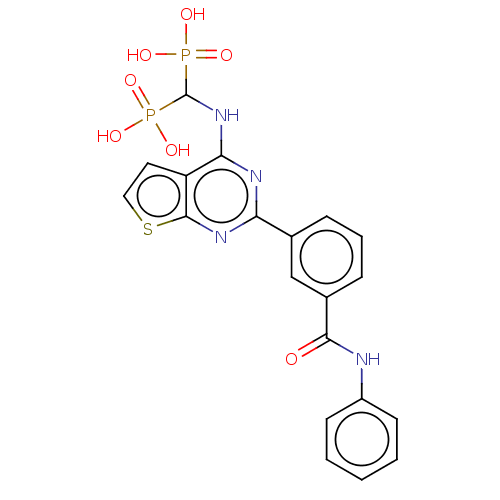 (CHEMBL4465832 | US11279719, Example I-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520639 (CHEMBL4465832 | US11279719, Example I-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520641 (CHEMBL4455060 | US11279719, Example I-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520637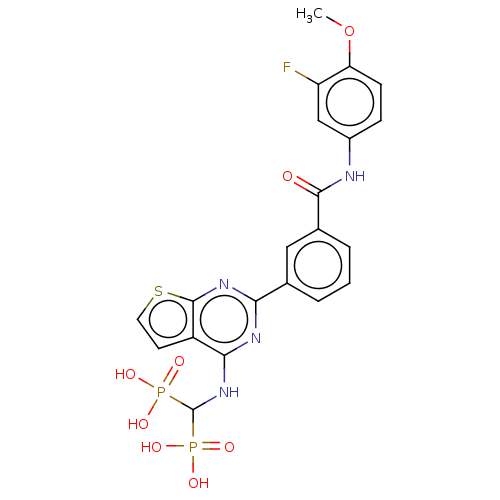 (CHEMBL4577077 | US11279719, Example I-37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520643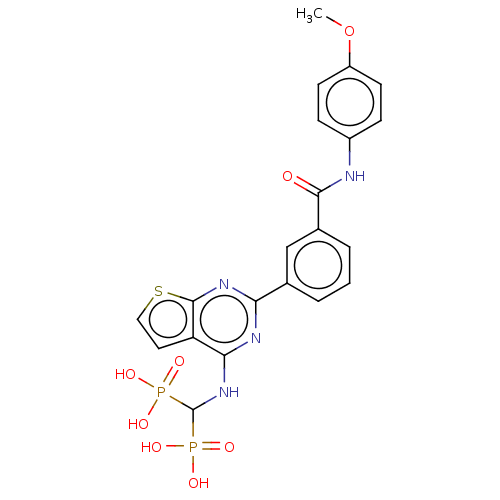 (CHEMBL4538679 | US11279719, Example I-39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520645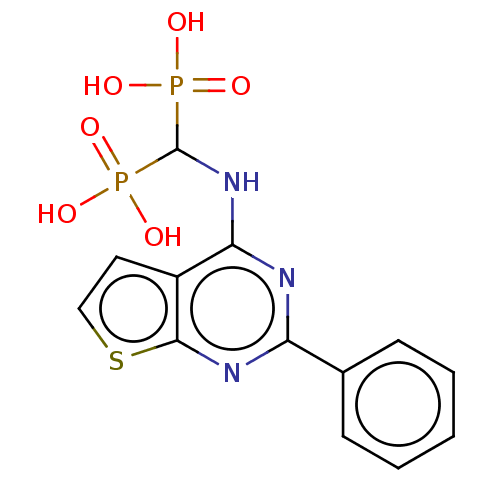 (CHEMBL2347859 | US11279719, Example C-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520646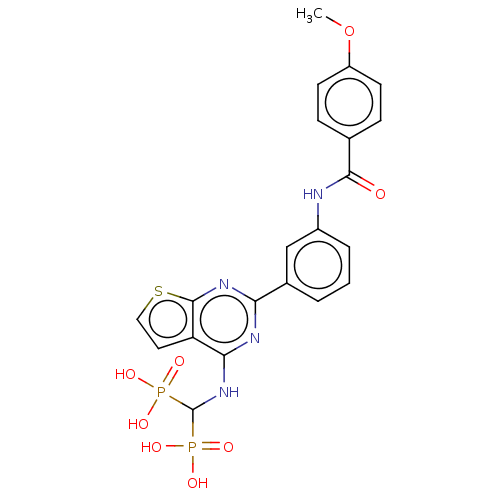 (CHEMBL4564934 | US11279719, Example I-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520637 (CHEMBL4577077 | US11279719, Example I-37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520646 (CHEMBL4564934 | US11279719, Example I-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520642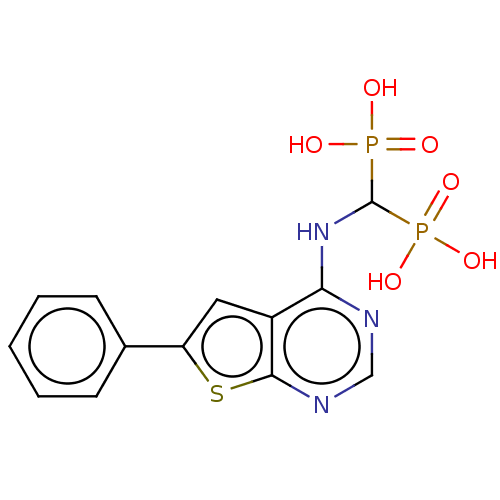 (CHEMBL2347861 | US11279719, Example C-10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520638 (CHEMBL4471037 | US11279719, Example I-18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520642 (CHEMBL2347861 | US11279719, Example C-10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25270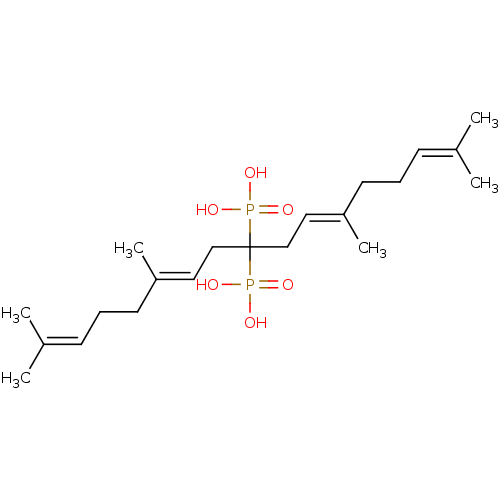 ([(6E,11E)-2,6,12,16-tetramethyl-9-phosphonoheptade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520645 (CHEMBL2347859 | US11279719, Example C-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25270 ([(6E,11E)-2,6,12,16-tetramethyl-9-phosphonoheptade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520644 (CHEMBL2347858 | US11279719, Example C-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520644 (CHEMBL2347858 | US11279719, Example C-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520640 (CHEMBL4472025 | US11279719, Example I-34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520646 (CHEMBL4564934 | US11279719, Example I-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520643 (CHEMBL4538679 | US11279719, Example I-39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520637 (CHEMBL4577077 | US11279719, Example I-37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50443054 (CHEMBL3087934 | US11279719, Example C-12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520641 (CHEMBL4455060 | US11279719, Example I-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520638 (CHEMBL4471037 | US11279719, Example I-18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50520639 (CHEMBL4465832 | US11279719, Example I-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50098378 ((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... | J Med Chem 61: 6904-6917 (2018) Article DOI: 10.1021/acs.jmedchem.8b00886 BindingDB Entry DOI: 10.7270/Q2CJ8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||