 Found 72 hits Enz. Inhib. hit(s) with all data for entry = 50009084
Found 72 hits Enz. Inhib. hit(s) with all data for entry = 50009084 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50254908
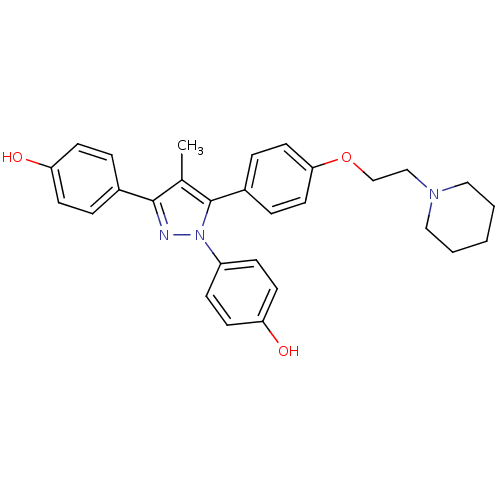
(4-[1-(4-hydroxyphenyl)-4-methyl-5-{4-[2-(piperidin...)Show SMILES Cc1c(nn(c1-c1ccc(OCCN2CCCCC2)cc1)-c1ccc(O)cc1)-c1ccc(O)cc1 Show InChI InChI=1S/C29H31N3O3/c1-21-28(22-5-11-25(33)12-6-22)30-32(24-9-13-26(34)14-10-24)29(21)23-7-15-27(16-8-23)35-20-19-31-17-3-2-4-18-31/h5-16,33-34H,2-4,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha in human MCF7 cells |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525255
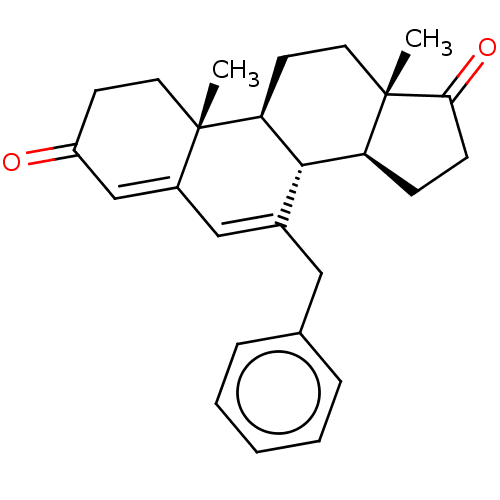
(CHEMBL3754505)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C(Cc2ccccc2)=CC2=CC(=O)CC[C@]12C |r,c:24,t:26| Show InChI InChI=1S/C26H30O2/c1-25-12-10-20(27)16-19(25)15-18(14-17-6-4-3-5-7-17)24-21-8-9-23(28)26(21,2)13-11-22(24)25/h3-7,15-16,21-22,24H,8-14H2,1-2H3/t21-,22-,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525268
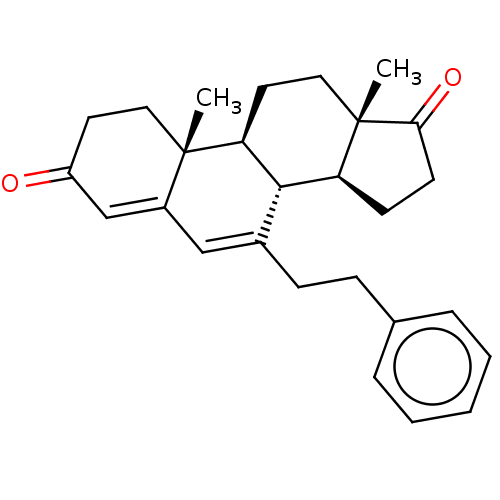
(CHEMBL3753089)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C(CCc2ccccc2)=CC2=CC(=O)CC[C@]12C |r,c:25,t:27| Show InChI InChI=1S/C27H32O2/c1-26-14-12-21(28)17-20(26)16-19(9-8-18-6-4-3-5-7-18)25-22-10-11-24(29)27(22,2)15-13-23(25)26/h3-7,16-17,22-23,25H,8-15H2,1-2H3/t22-,23-,25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9454
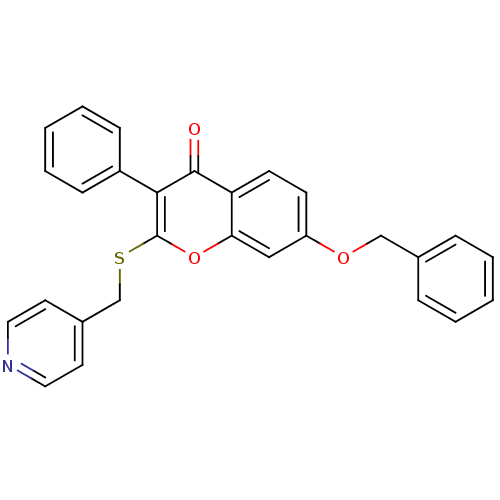
(7-(benzyloxy)-3-phenyl-2-[(pyridin-4-ylmethyl)sulf...)Show SMILES O=c1c(-c2ccccc2)c(SCc2ccncc2)oc2cc(OCc3ccccc3)ccc12 Show InChI InChI=1S/C28H21NO3S/c30-27-24-12-11-23(31-18-20-7-3-1-4-8-20)17-25(24)32-28(26(27)22-9-5-2-6-10-22)33-19-21-13-15-29-16-14-21/h1-17H,18-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta[3H]]androst-4-ene-3,17-dione as substrate after 15 mins in presence of NADPH by liquid... |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525262
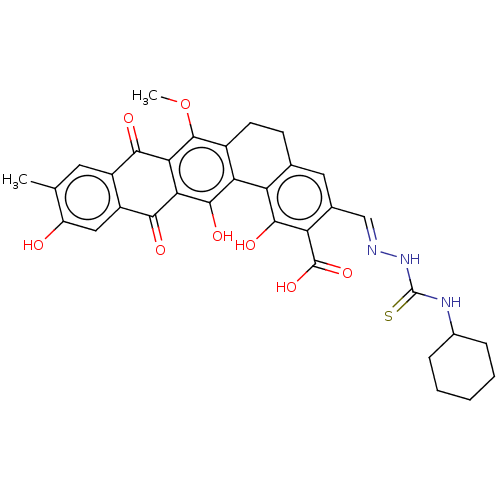
(CHEMBL4444347)Show SMILES COc1c2CCc3cc(\C=N\NC(=S)NC4CCCCC4)c(C(O)=O)c(O)c3-c2c(O)c2C(=O)c3cc(O)c(C)cc3C(=O)c12 Show InChI InChI=1S/C33H31N3O8S/c1-14-10-19-20(12-21(14)37)27(38)25-26(28(19)39)31(44-2)18-9-8-15-11-16(13-34-36-33(45)35-17-6-4-3-5-7-17)23(32(42)43)29(40)22(15)24(18)30(25)41/h10-13,17,37,40-41H,3-9H2,1-2H3,(H,42,43)(H2,35,36,45)/b34-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) using [3H]E1S as substrate after 20 mins by scintillation counting method |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50254908

(4-[1-(4-hydroxyphenyl)-4-methyl-5-{4-[2-(piperidin...)Show SMILES Cc1c(nn(c1-c1ccc(OCCN2CCCCC2)cc1)-c1ccc(O)cc1)-c1ccc(O)cc1 Show InChI InChI=1S/C29H31N3O3/c1-21-28(22-5-11-25(33)12-6-22)30-32(24-9-13-26(34)14-10-24)29(21)23-7-15-27(16-8-23)35-20-19-31-17-3-2-4-18-31/h5-16,33-34H,2-4,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of ERbeta in human MCF7 cells |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9461
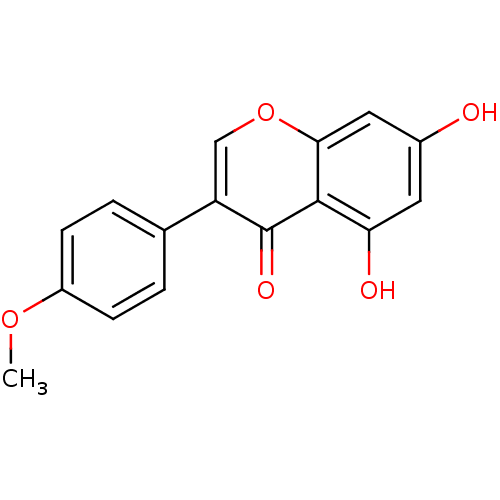
(5,7-dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C16H12O5/c1-20-11-4-2-9(3-5-11)12-8-21-14-7-10(17)6-13(18)15(14)16(12)19/h2-8,17-18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate after 15 mins in presence of NADPH by liquid scin... |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50370691
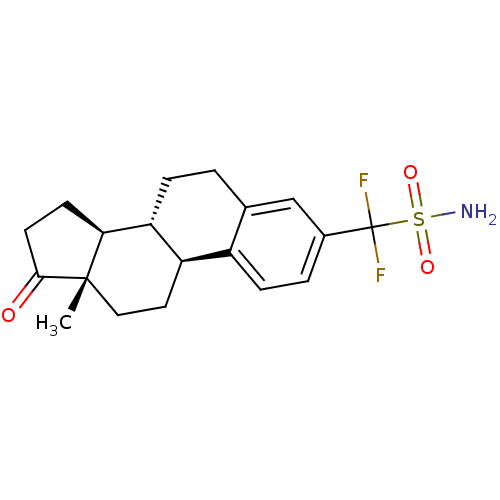
(CHEMBL1628004)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(ccc34)C(F)(F)S(N)(=O)=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H23F2NO3S/c1-18-9-8-14-13-5-3-12(19(20,21)26(22,24)25)10-11(13)2-4-15(14)16(18)6-7-17(18)23/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,22,24,25)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50073853
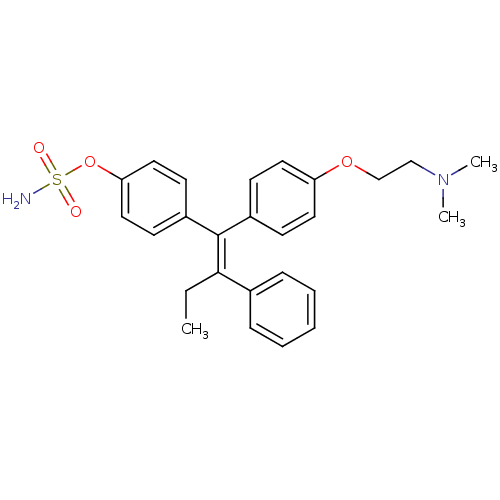
(CHEMBL33862 | Sulfamic acid 4-{(E)-1-[4-(2-dimethy...)Show SMILES CC\C(=C(\c1ccc(OCCN(C)C)cc1)c1ccc(OS(N)(=O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C26H30N2O4S/c1-4-25(20-8-6-5-7-9-20)26(22-12-16-24(17-13-22)32-33(27,29)30)21-10-14-23(15-11-21)31-19-18-28(2)3/h5-17H,4,18-19H2,1-3H3,(H2,27,29,30)/b26-25+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50073852
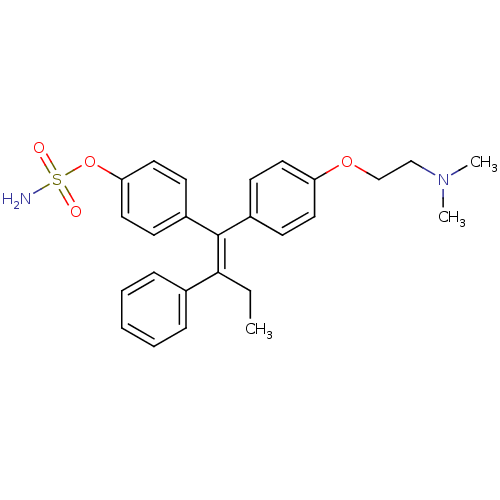
(CHEMBL32172 | Sulfamic acid 4-{(Z)-1-[4-(2-dimethy...)Show SMILES CC\C(=C(/c1ccc(OCCN(C)C)cc1)c1ccc(OS(N)(=O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C26H30N2O4S/c1-4-25(20-8-6-5-7-9-20)26(22-12-16-24(17-13-22)32-33(27,29)30)21-10-14-23(15-11-21)31-19-18-28(2)3/h5-17H,4,18-19H2,1-3H3,(H2,27,29,30)/b26-25- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50525253
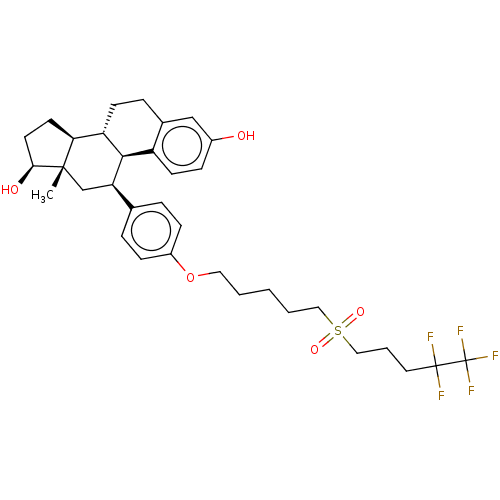
(CHEMBL4518283)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)C[C@H](c1ccc(OCCCCCS(=O)(=O)CCCC(F)(F)C(F)(F)F)cc1)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C34H43F5O5S/c1-32-21-28(31-26-13-9-24(40)20-23(26)8-12-27(31)29(32)14-15-30(32)41)22-6-10-25(11-7-22)44-17-3-2-4-18-45(42,43)19-5-16-33(35,36)34(37,38)39/h6-7,9-11,13,20,27-31,40-41H,2-5,8,12,14-19,21H2,1H3/t27-,28+,29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha in human MCF7 cells after 36 hrs by Western blot analysis |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118551

(CHEMBL137392 | Sulfamic acid 2-tert-butyl-4-oxo-4H...)Show InChI InChI=1S/C13H15NO5S/c1-13(2,3)12-7-10(15)9-6-8(19-20(14,16)17)4-5-11(9)18-12/h4-7H,1-3H3,(H2,14,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human sulfatase using 4-methylumbelliferyl sulfate as substrate after 60 mins |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118567
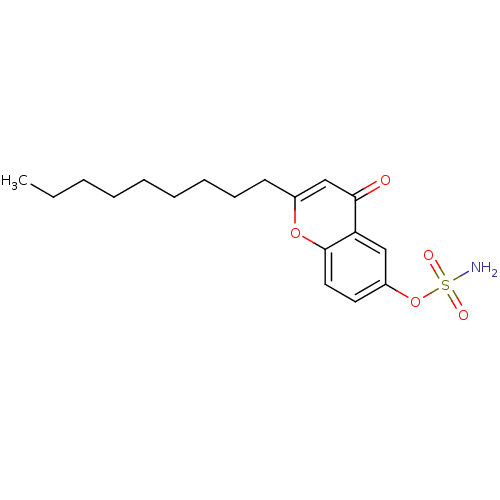
(CHEMBL137692 | Sulfamic acid 2-nonyl-4-oxo-4H-chro...)Show InChI InChI=1S/C18H25NO5S/c1-2-3-4-5-6-7-8-9-14-13-17(20)16-12-15(24-25(19,21)22)10-11-18(16)23-14/h10-13H,2-9H2,1H3,(H2,19,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human sulfatase using 4-methylumbelliferyl sulfate as substrate after 60 mins |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525257

(CHEMBL4520995)Show SMILES [O-][N+](=O)c1ccc(cc1)N(Cc1ccc(Br)cc1)n1cnnc1 Show InChI InChI=1S/C15H12BrN5O2/c16-13-3-1-12(2-4-13)9-20(19-10-17-18-11-19)14-5-7-15(8-6-14)21(22)23/h1-8,10-11H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate by liquid scintillation counting method |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50369432

(CHEMBL1627465)Show SMILES CC(C)(C)c1ccc(C[C@]2(O)CC[C@H]3[C@@H]4CCc5cc(OS(N)(=O)=O)ccc5[C@H]4CC[C@]23C)cc1 Show InChI InChI=1S/C29H39NO4S/c1-27(2,3)21-8-5-19(6-9-21)18-29(31)16-14-26-25-11-7-20-17-22(34-35(30,32)33)10-12-23(20)24(25)13-15-28(26,29)4/h5-6,8-10,12,17,24-26,31H,7,11,13-16,18H2,1-4H3,(H2,30,32,33)/t24-,25-,26+,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) using [3H]E1S as substrate after 18 hrs |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525260
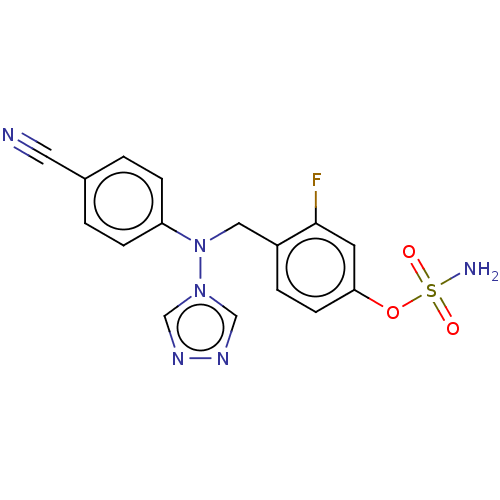
(CHEMBL4465348)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2cnnc2)c(F)c1 Show InChI InChI=1S/C16H13FN6O3S/c17-16-7-15(26-27(19,24)25)6-3-13(16)9-23(22-10-20-21-11-22)14-4-1-12(8-18)2-5-14/h1-7,10-11H,9H2,(H2,19,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121079

(CHEMBL3622064)Show SMILES NS(=O)(=O)Oc1ccc2cc(CN(c3ccc(cc3)C#N)n3cnnc3)ccc2c1Br Show InChI InChI=1S/C20H15BrN6O3S/c21-20-18-7-3-15(9-16(18)4-8-19(20)30-31(23,28)29)11-27(26-12-24-25-13-26)17-5-1-14(10-22)2-6-17/h1-9,12-13H,11H2,(H2,23,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121105
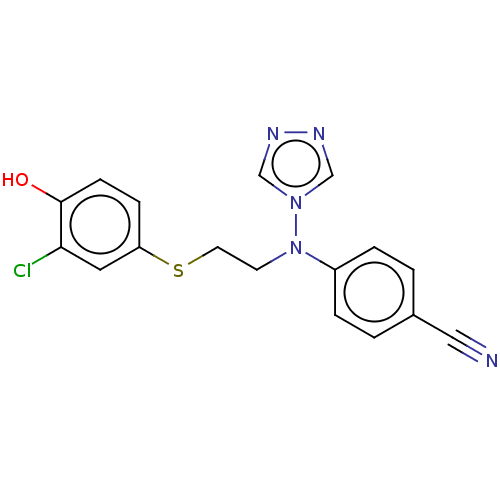
(CHEMBL3622055)Show InChI InChI=1S/C17H14ClN5OS/c18-16-9-15(5-6-17(16)24)25-8-7-23(22-11-20-21-12-22)14-3-1-13(10-19)2-4-14/h1-6,9,11-12,24H,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118560
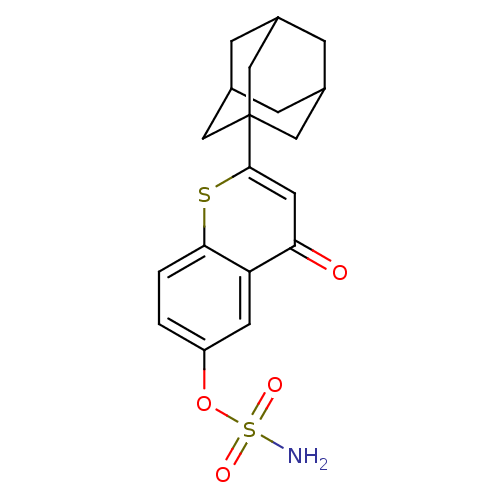
(CHEMBL262050 | Sulfamic acid 2-adamantan-1-yl-4-ox...)Show SMILES NS(=O)(=O)Oc1ccc2sc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:22:25:17.18.19,23:18:25:24.22.21,THB:21:22:17:25.20.19,21:20:17:24.22.23| Show InChI InChI=1S/C19H21NO4S2/c20-26(22,23)24-14-1-2-17-15(6-14)16(21)7-18(25-17)19-8-11-3-12(9-19)5-13(4-11)10-19/h1-2,6-7,11-13H,3-5,8-10H2,(H2,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) using 4-MUS as substrate |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50369431
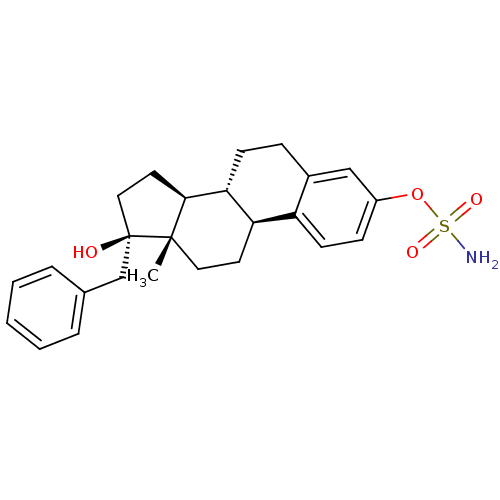
(CHEMBL1627878)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CC[C@@]2(O)Cc1ccccc1 Show InChI InChI=1S/C25H31NO4S/c1-24-13-11-21-20-10-8-19(30-31(26,28)29)15-18(20)7-9-22(21)23(24)12-14-25(24,27)16-17-5-3-2-4-6-17/h2-6,8,10,15,21-23,27H,7,9,11-14,16H2,1H3,(H2,26,28,29)/t21-,22-,23+,24+,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) using [3H]E1S as substrate after 18 hrs |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10016

(4-{[(4-bromophenyl)methyl](4H-1,2,4-triazol-4-yl)a...)Show InChI InChI=1S/C16H12BrN5/c17-15-5-1-14(2-6-15)10-22(21-11-19-20-12-21)16-7-3-13(9-18)4-8-16/h1-8,11-12H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate by liquid scintillation counting method |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50047259

(4-(1-(1H-imidazol-1-yl)vinyl)benzonitrile | 4-(1-I...)Show InChI InChI=1S/C12H9N3/c1-10(15-7-6-14-9-15)12-4-2-11(8-13)3-5-12/h2-7,9H,1H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10020

((2-bromo-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl...)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2cnnc2)cc1Br Show InChI InChI=1S/C16H13BrN6O3S/c17-15-7-13(3-6-16(15)26-27(19,24)25)9-23(22-10-20-21-11-22)14-4-1-12(8-18)2-5-14/h1-7,10-11H,9H2,(H2,19,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50369432

(CHEMBL1627465)Show SMILES CC(C)(C)c1ccc(C[C@]2(O)CC[C@H]3[C@@H]4CCc5cc(OS(N)(=O)=O)ccc5[C@H]4CC[C@]23C)cc1 Show InChI InChI=1S/C29H39NO4S/c1-27(2,3)21-8-5-19(6-9-21)18-29(31)16-14-26-25-11-7-20-17-22(34-35(30,32)33)10-12-23(20)24(25)13-15-28(26,29)4/h5-6,8-10,12,17,24-26,31H,7,11,13-16,18H2,1-4H3,(H2,30,32,33)/t24-,25-,26+,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) using [3H]DHEAS as substrate after 18 hrs |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121078
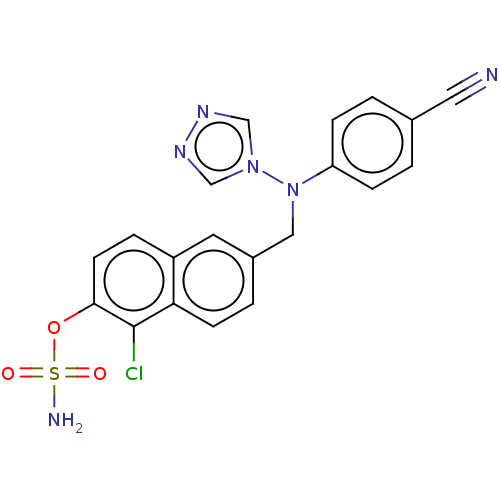
(CHEMBL3622063)Show SMILES NS(=O)(=O)Oc1ccc2cc(CN(c3ccc(cc3)C#N)n3cnnc3)ccc2c1Cl Show InChI InChI=1S/C20H15ClN6O3S/c21-20-18-7-3-15(9-16(18)4-8-19(20)30-31(23,28)29)11-27(26-12-24-25-13-26)17-5-1-14(10-22)2-6-17/h1-9,12-13H,11H2,(H2,23,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10019
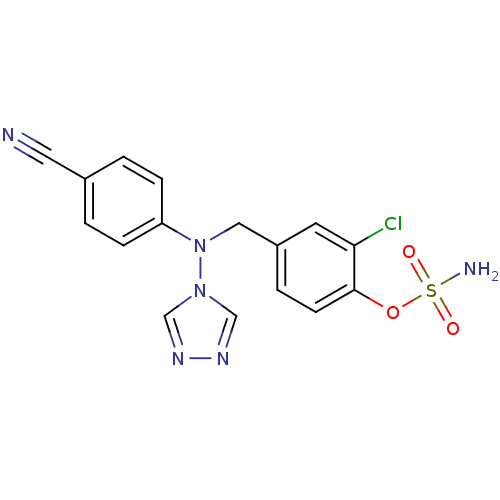
((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2cnnc2)cc1Cl Show InChI InChI=1S/C16H13ClN6O3S/c17-15-7-13(3-6-16(15)26-27(19,24)25)9-23(22-10-20-21-11-22)14-4-1-12(8-18)2-5-14/h1-7,10-11H,9H2,(H2,19,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525260

(CHEMBL4465348)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2cnnc2)c(F)c1 Show InChI InChI=1S/C16H13FN6O3S/c17-16-7-15(26-27(19,24)25)6-3-13(16)9-23(22-10-20-21-11-22)14-4-1-12(8-18)2-5-14/h1-7,10-11H,9H2,(H2,19,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50041611
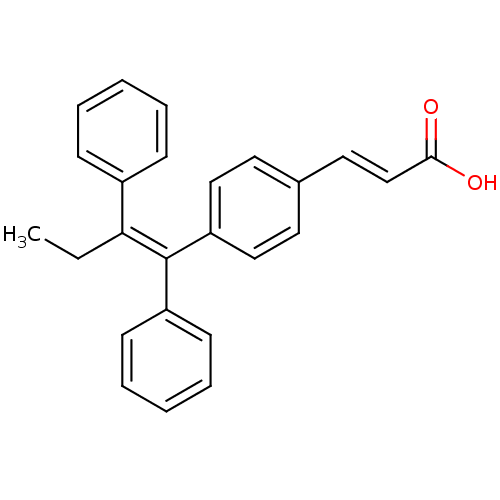
((2E)-3-{4-[(1E)-1,2-DIPHENYLBUT-1-ENYL]PHENYL}ACRY...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O2/c1-2-23(20-9-5-3-6-10-20)25(21-11-7-4-8-12-21)22-16-13-19(14-17-22)15-18-24(26)27/h3-18H,2H2,1H3,(H,26,27)/b18-15+,25-23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of Fluormone-ES2 binding affinity to ERalpha (unknown origin) after 2 hrs by fluorescent polarization based competition binding assay |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50121196
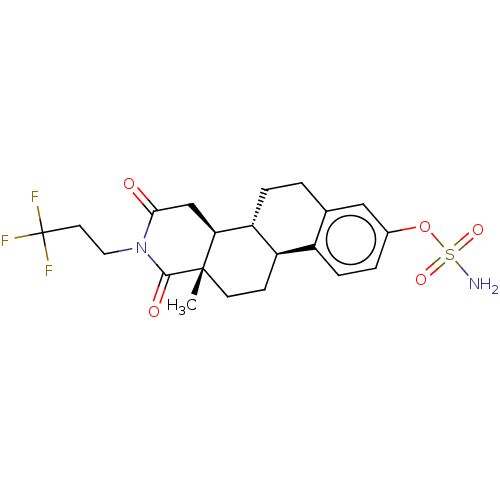
(CHEMBL3622029)Show SMILES [H][C@]12CC[C@]3(C)C(=O)N(CCC(F)(F)F)C(=O)C[C@@]3([H])[C@]1([H])CCc1cc(OS(N)(=O)=O)ccc21 |r| Show InChI InChI=1S/C21H25F3N2O5S/c1-20-7-6-15-14-5-3-13(31-32(25,29)30)10-12(14)2-4-16(15)17(20)11-18(27)26(19(20)28)9-8-21(22,23)24/h3,5,10,15-17H,2,4,6-9,11H2,1H3,(H2,25,29,30)/t15-,16-,17+,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50525263
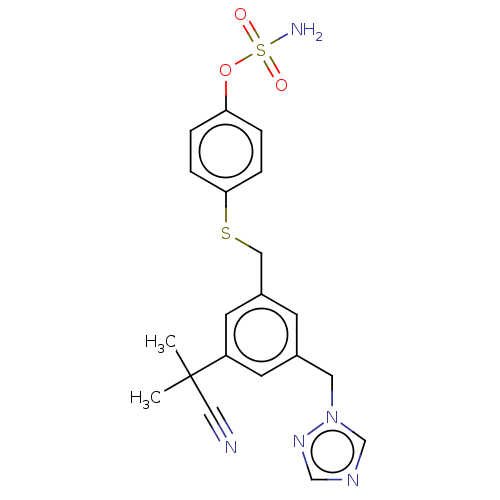
(CHEMBL4444074)Show SMILES CC(C)(C#N)c1cc(CSc2ccc(OS(N)(=O)=O)cc2)cc(Cn2cncn2)c1 Show InChI InChI=1S/C20H21N5O3S2/c1-20(2,12-21)17-8-15(10-25-14-23-13-24-25)7-16(9-17)11-29-19-5-3-18(4-6-19)28-30(22,26)27/h3-9,13-14H,10-11H2,1-2H3,(H2,22,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50369431

(CHEMBL1627878)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CC[C@@]2(O)Cc1ccccc1 Show InChI InChI=1S/C25H31NO4S/c1-24-13-11-21-20-10-8-19(30-31(26,28)29)15-18(20)7-9-22(21)23(24)12-14-25(24,27)16-17-5-3-2-4-6-17/h2-6,8,10,15,21-23,27H,7,9,11-14,16H2,1H3,(H2,26,28,29)/t21-,22-,23+,24+,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) using [3H]DHEAS as substrate after 18 hrs |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525261
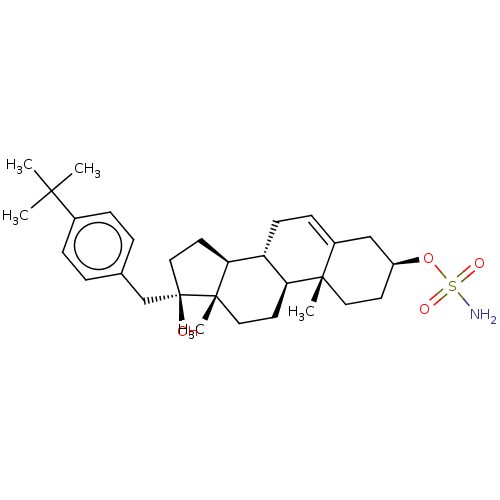
(CHEMBL4436482)Show SMILES [H][C@@]12CC[C@@](O)(Cc3ccc(cc3)C(C)(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OS(N)(=O)=O |r,t:29| Show InChI InChI=1S/C30H45NO4S/c1-27(2,3)21-8-6-20(7-9-21)19-30(32)17-14-26-24-11-10-22-18-23(35-36(31,33)34)12-15-28(22,4)25(24)13-16-29(26,30)5/h6-10,23-26,32H,11-19H2,1-5H3,(H2,31,33,34)/t23-,24+,25-,26-,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) expressed in HEK293 cells using [3H]DHEAS as substrate after 2 hrs by liquid scintillation counting method |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM10019

((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2cnnc2)cc1Cl Show InChI InChI=1S/C16H13ClN6O3S/c17-15-7-13(3-6-16(15)26-27(19,24)25)9-23(22-10-20-21-11-22)14-4-1-12(8-18)2-5-14/h1-7,10-11H,9H2,(H2,19,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50363594
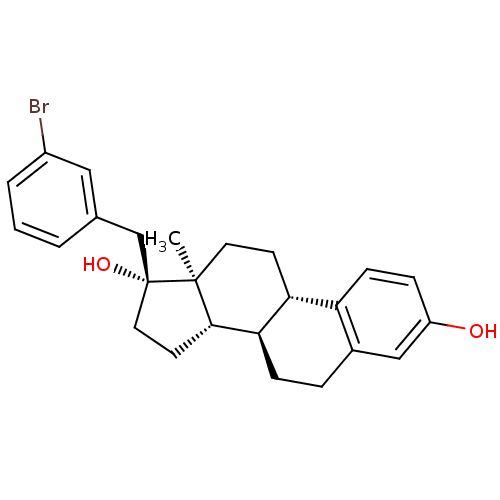
(CHEMBL1627429)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)Cc1cccc(Br)c1 Show InChI InChI=1S/C25H29BrO2/c1-24-11-9-21-20-8-6-19(27)14-17(20)5-7-22(21)23(24)10-12-25(24,28)15-16-3-2-4-18(26)13-16/h2-4,6,8,13-14,21-23,27-28H,5,7,9-12,15H2,1H3/t21-,22-,23+,24+,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50121078

(CHEMBL3622063)Show SMILES NS(=O)(=O)Oc1ccc2cc(CN(c3ccc(cc3)C#N)n3cnnc3)ccc2c1Cl Show InChI InChI=1S/C20H15ClN6O3S/c21-20-18-7-3-15(9-16(18)4-8-19(20)30-31(23,28)29)11-27(26-12-24-25-13-26)17-5-1-14(10-22)2-6-17/h1-9,12-13H,11H2,(H2,23,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50366532

(CHEMBL518966)Show SMILES CC(C)(C)c1ccc(C[C@]2(O)CC[C@H]3[C@@H]4CCc5cc(O)ccc5[C@H]4CC[C@]23C)cc1 Show InChI InChI=1S/C29H38O2/c1-27(2,3)21-8-5-19(6-9-21)18-29(31)16-14-26-25-11-7-20-17-22(30)10-12-23(20)24(25)13-15-28(26,29)4/h5-6,8-10,12,17,24-26,30-31H,7,11,13-16,18H2,1-4H3/t24-,25-,26+,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50121058
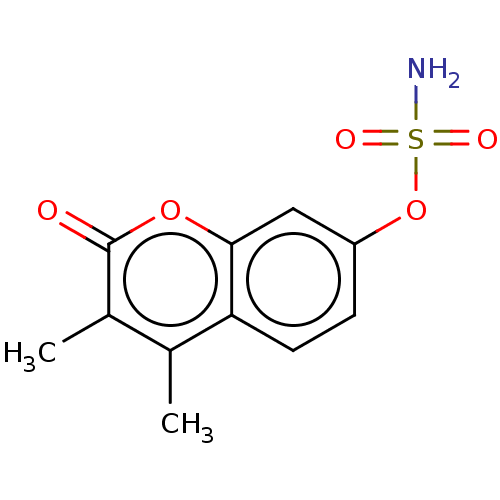
(CHEMBL275331)Show InChI InChI=1S/C11H11NO5S/c1-6-7(2)11(13)16-10-5-8(3-4-9(6)10)17-18(12,14)15/h3-5H,1-2H3,(H2,12,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human sulfatase using 4-methylumbelliferyl sulfate as substrate after 60 mins |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50525266
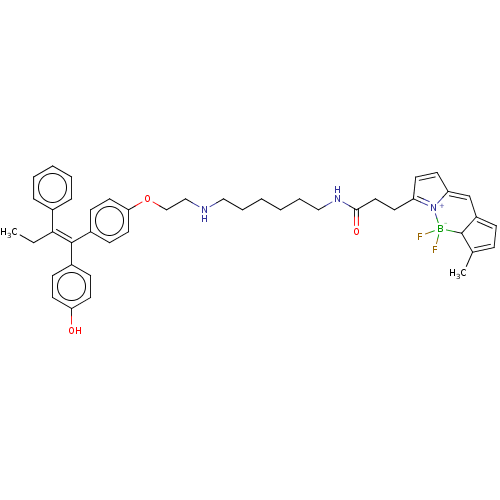
(CHEMBL4547986)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCNCCCCCCNC(=O)CCC2=[N+]3C(C=C2)=CC2=CC=C(C)C2[B-]3(F)F)cc1)c1ccccc1 |c:34,36,t:31,38,40| Show InChI InChI=1S/C44H50BF2N3O3/c1-3-41(33-11-7-6-8-12-33)43(34-15-22-39(51)23-16-34)35-17-24-40(25-18-35)53-30-29-48-27-9-4-5-10-28-49-42(52)26-21-37-19-20-38-31-36-14-13-32(2)44(36)45(46,47)50(37)38/h6-8,11-20,22-25,31,44,48,51H,3-5,9-10,21,26-30H2,1-2H3,(H,49,52)/b43-41- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) after 18 hrs by dual luciferase reporter gene assay |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM10020

((2-bromo-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl...)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2cnnc2)cc1Br Show InChI InChI=1S/C16H13BrN6O3S/c17-15-7-13(3-6-16(15)26-27(19,24)25)9-23(22-10-20-21-11-22)14-4-1-12(8-18)2-5-14/h1-7,10-11H,9H2,(H2,19,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50097366

(4-((1H-imidazol-1-yl)methyl)-1-nitro-4aH-xanthen-9...)Show SMILES [O-][N+](=O)c1ccc(Cn2ccnc2)c2oc3ccccc3c(=O)c12 Show InChI InChI=1S/C17H11N3O4/c21-16-12-3-1-2-4-14(12)24-17-11(9-19-8-7-18-10-19)5-6-13(15(16)17)20(22)23/h1-8,10H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase using [1beta,2beta3H]-]testosterone as substrate |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525256

(CHEMBL4540847)Show SMILES Oc1ccc2[C@H](C[C@@H](Oc2c1)c1ccccc1)n1ccnc1 |r| Show InChI InChI=1S/C18H16N2O2/c21-14-6-7-15-16(20-9-8-19-12-20)11-17(22-18(15)10-14)13-4-2-1-3-5-13/h1-10,12,16-17,21H,11H2/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
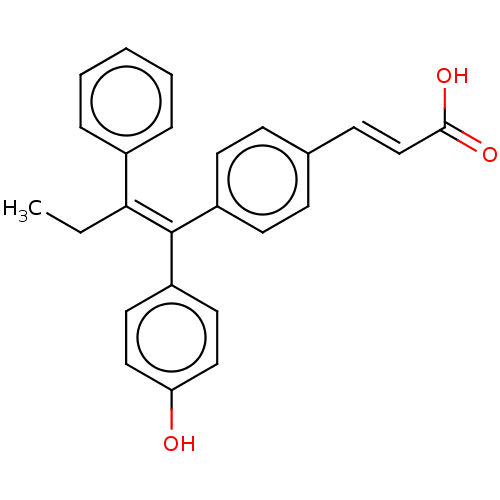
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of Fluormone-ES2 binding affinity to ERalpha (unknown origin) after 2 hrs by fluorescent polarization based competition binding assay |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50097373
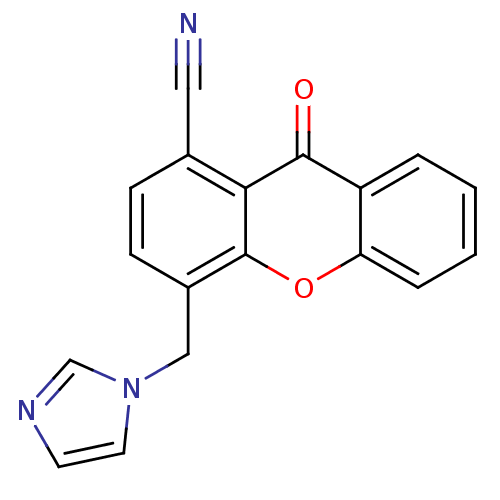
(4-((1H-imidazol-1-yl)methyl)-9-oxo-9H-xanthene-1-c...)Show InChI InChI=1S/C18H11N3O2/c19-9-12-5-6-13(10-21-8-7-20-11-21)18-16(12)17(22)14-3-1-2-4-15(14)23-18/h1-8,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase using [1beta,2beta3H]-]testosterone as substrate |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525261

(CHEMBL4436482)Show SMILES [H][C@@]12CC[C@@](O)(Cc3ccc(cc3)C(C)(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@H](CC[C@]12C)OS(N)(=O)=O |r,t:29| Show InChI InChI=1S/C30H45NO4S/c1-27(2,3)21-8-6-20(7-9-21)19-30(32)17-14-26-24-11-10-22-18-23(35-36(31,33)34)12-15-28(22,4)25(24)13-16-29(26,30)5/h6-10,23-26,32H,11-19H2,1-5H3,(H2,31,33,34)/t23-,24+,25-,26-,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) expressed in HEK293 cells using [3H]E1S as substrate after 2 hrs by liquid scintillation counting method |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525258
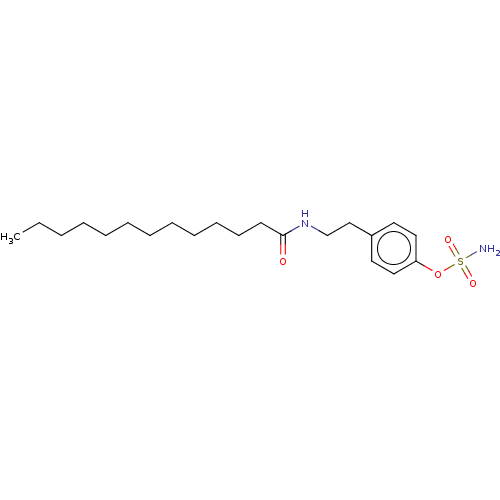
(CHEMBL4529949)Show InChI InChI=1S/C21H36N2O4S/c1-2-3-4-5-6-7-8-9-10-11-12-21(24)23-18-17-19-13-15-20(16-14-19)27-28(22,25)26/h13-16H,2-12,17-18H2,1H3,(H,23,24)(H2,22,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of sulfatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525270

(CHEMBL4435618)Show InChI InChI=1S/C21H36N2O4S/c1-2-3-4-5-6-7-8-9-10-11-12-13-21(24)23-18-19-14-16-20(17-15-19)27-28(22,25)26/h14-17H,2-13,18H2,1H3,(H,23,24)(H2,22,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome sulfatase using estrone sulfate as substrate |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50525254

(CHEMBL4475271)Show SMILES [H][C@@]12C[C@@H](C\C=C\CNC3=C4C[C@@H](C)C[C@H](OC)[C@H](O)[C@@H](C)\C=C(C)\[C@H](OC(N)=O)[C@@H](OC)\C=C/C=C(C)/C(=O)NC(=CC3=O)C4=O)[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r,c:9,22,33,35,t:41| Show InChI InChI=1S/C50H67N3O10/c1-27-21-37-43(52-20-9-8-12-32-25-38-36-16-14-31-24-33(54)15-17-34(31)35(36)18-19-50(38,5)47(32)58)40(55)26-39(45(37)57)53-48(59)28(2)11-10-13-41(61-6)46(63-49(51)60)30(4)23-29(3)44(56)42(22-27)62-7/h8-11,13,15,17,23-24,26-27,29,32,35-36,38,41-42,44,46-47,52,54,56,58H,12,14,16,18-22,25H2,1-7H3,(H2,51,60)(H,53,59)/b9-8+,13-10-,28-11+,30-23+/t27-,29+,32-,35-,36-,38+,41+,42+,44-,46+,47+,50+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of Estrogen receptor (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525267

(CHEMBL4583456)Show SMILES COc1ccc2[C@H](C[C@@H](Oc2c1)c1ccccc1)n1ccnc1 |r| Show InChI InChI=1S/C19H18N2O2/c1-22-15-7-8-16-17(21-10-9-20-13-21)12-18(23-19(16)11-15)14-5-3-2-4-6-14/h2-11,13,17-18H,12H2,1H3/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50525254

(CHEMBL4475271)Show SMILES [H][C@@]12C[C@@H](C\C=C\CNC3=C4C[C@@H](C)C[C@H](OC)[C@H](O)[C@@H](C)\C=C(C)\[C@H](OC(N)=O)[C@@H](OC)\C=C/C=C(C)/C(=O)NC(=CC3=O)C4=O)[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r,c:9,22,33,35,t:41| Show InChI InChI=1S/C50H67N3O10/c1-27-21-37-43(52-20-9-8-12-32-25-38-36-16-14-31-24-33(54)15-17-34(31)35(36)18-19-50(38,5)47(32)58)40(55)26-39(45(37)57)53-48(59)28(2)11-10-13-41(61-6)46(63-49(51)60)30(4)23-29(3)44(56)42(22-27)62-7/h8-11,13,15,17,23-24,26-27,29,32,35-36,38,41-42,44,46-47,52,54,56,58H,12,14,16,18-22,25H2,1-7H3,(H2,51,60)(H,53,59)/b9-8+,13-10-,28-11+,30-23+/t27-,29+,32-,35-,36-,38+,41+,42+,44-,46+,47+,50+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of HER2 (unknown origin) |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50525252
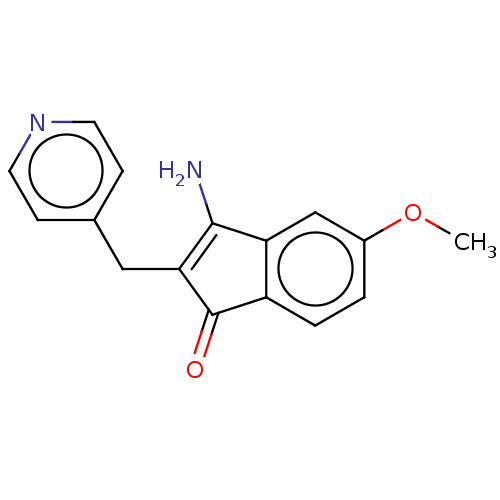
(CHEMBL1997442)Show InChI InChI=1S/C16H14N2O2/c1-20-11-2-3-12-13(9-11)15(17)14(16(12)19)8-10-4-6-18-7-5-10/h2-7,9H,8,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate after 15 mins in presence of NADPH by liquid scin... |
Eur J Med Chem 177: 116-143 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.023
BindingDB Entry DOI: 10.7270/Q2W099B9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data