 Found 37 hits Enz. Inhib. hit(s) with all data for entry = 50009999
Found 37 hits Enz. Inhib. hit(s) with all data for entry = 50009999 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534272
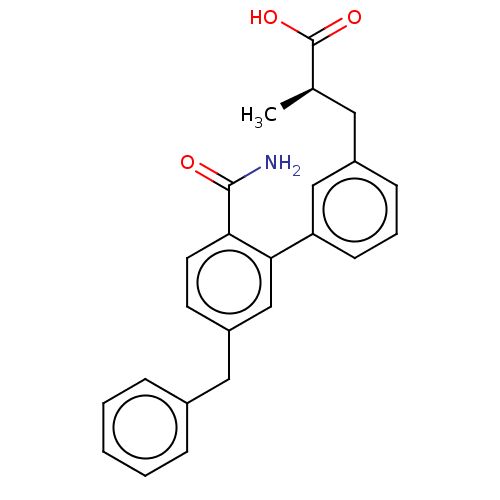
(CHEMBL4593409)Show SMILES C[C@H](Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H23NO3/c1-16(24(27)28)12-18-8-5-9-20(14-18)22-15-19(10-11-21(22)23(25)26)13-17-6-3-2-4-7-17/h2-11,14-16H,12-13H2,1H3,(H2,25,26)(H,27,28)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534275
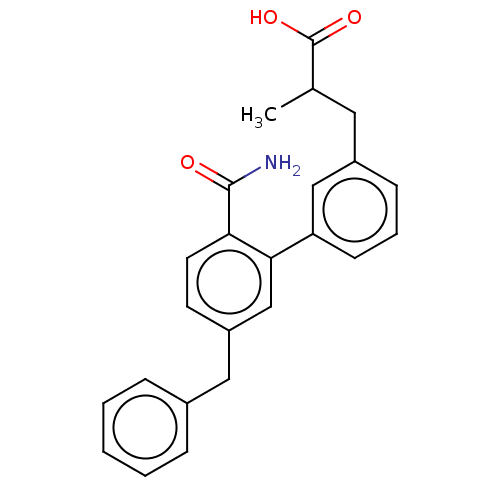
(CHEMBL4451830)Show SMILES CC(Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O Show InChI InChI=1S/C24H23NO3/c1-16(24(27)28)12-18-8-5-9-20(14-18)22-15-19(10-11-21(22)23(25)26)13-17-6-3-2-4-7-17/h2-11,14-16H,12-13H2,1H3,(H2,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534274
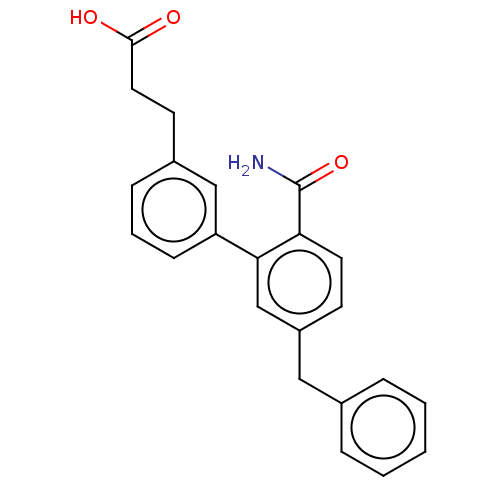
(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Group XIIB secretory phospholipase A2-like protein
(Homo sapiens) | BDBM50534272

(CHEMBL4593409)Show SMILES C[C@H](Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H23NO3/c1-16(24(27)28)12-18-8-5-9-20(14-18)22-15-19(10-11-21(22)23(25)26)13-17-6-3-2-4-7-17/h2-11,14-16H,12-13H2,1H3,(H2,25,26)(H,27,28)/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <14 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2 in human HepG2 cells |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534277
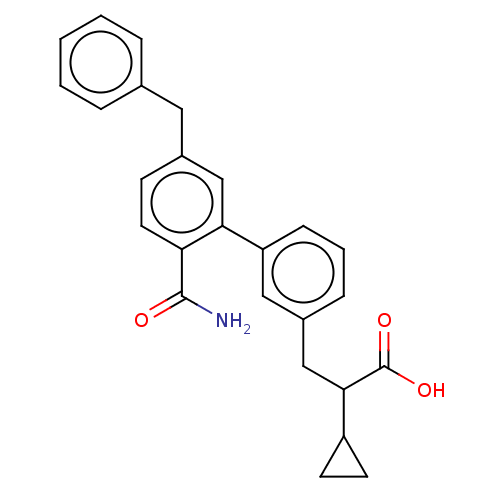
(CHEMBL4453126)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CC(C2CC2)C(O)=O)c1 Show InChI InChI=1S/C26H25NO3/c27-25(28)22-12-9-19(13-17-5-2-1-3-6-17)15-23(22)21-8-4-7-18(14-21)16-24(26(29)30)20-10-11-20/h1-9,12,14-15,20,24H,10-11,13,16H2,(H2,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534278
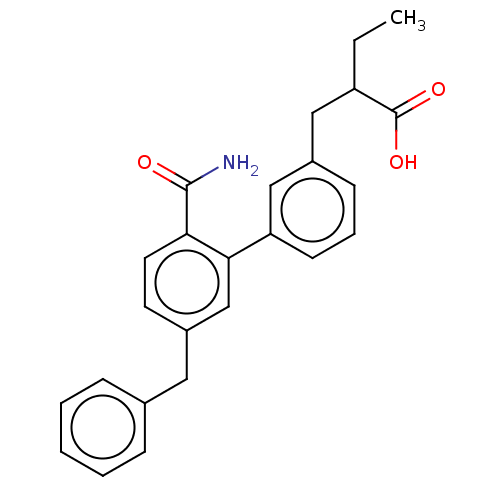
(CHEMBL4533403)Show SMILES CCC(Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O Show InChI InChI=1S/C25H25NO3/c1-2-20(25(28)29)14-18-9-6-10-21(15-18)23-16-19(11-12-22(23)24(26)27)13-17-7-4-3-5-8-17/h3-12,15-16,20H,2,13-14H2,1H3,(H2,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534276
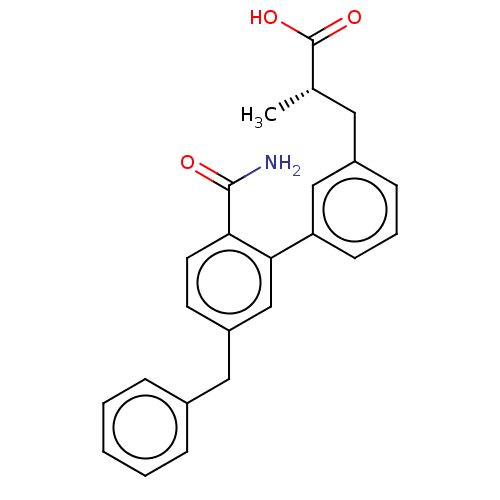
(CHEMBL4555962)Show SMILES C[C@@H](Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H23NO3/c1-16(24(27)28)12-18-8-5-9-20(14-18)22-15-19(10-11-21(22)23(25)26)13-17-6-3-2-4-7-17/h2-11,14-16H,12-13H2,1H3,(H2,25,26)(H,27,28)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group XIIB secretory phospholipase A2-like protein
(Homo sapiens) | BDBM50534272

(CHEMBL4593409)Show SMILES C[C@H](Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H23NO3/c1-16(24(27)28)12-18-8-5-9-20(14-18)22-15-19(10-11-21(22)23(25)26)13-17-6-3-2-4-7-17/h2-11,14-16H,12-13H2,1H3,(H2,25,26)(H,27,28)/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2 in atherosclerotic plaque from coronary artery disease patient |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534279
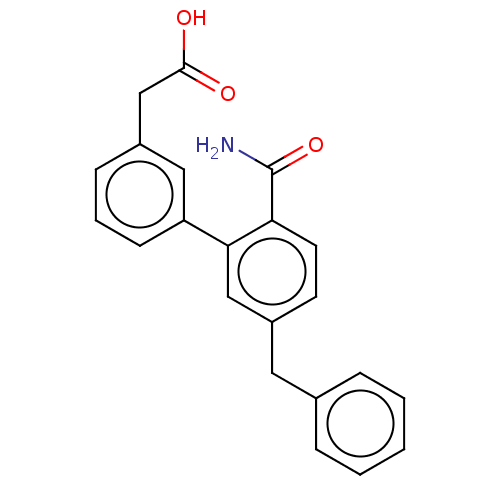
(CHEMBL4468927)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CC(O)=O)c1 Show InChI InChI=1S/C22H19NO3/c23-22(26)19-10-9-17(11-15-5-2-1-3-6-15)13-20(19)18-8-4-7-16(12-18)14-21(24)25/h1-10,12-13H,11,14H2,(H2,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534280
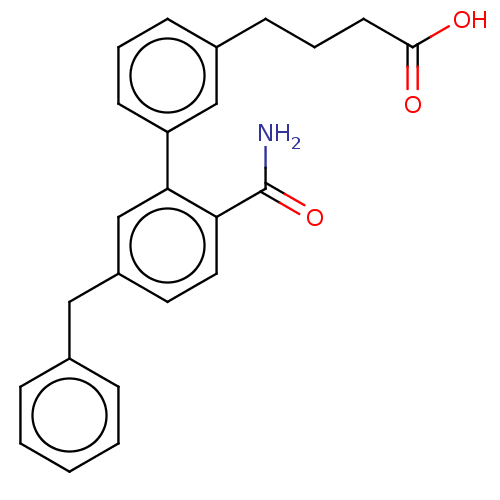
(CHEMBL4591759)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCCC(O)=O)c1 Show InChI InChI=1S/C24H23NO3/c25-24(28)21-13-12-19(14-17-6-2-1-3-7-17)16-22(21)20-10-4-8-18(15-20)9-5-11-23(26)27/h1-4,6-8,10,12-13,15-16H,5,9,11,14H2,(H2,25,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534271

(CHEMBL4469134)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(OCC(O)=O)c1 Show InChI InChI=1S/C22H19NO4/c23-22(26)19-10-9-16(11-15-5-2-1-3-6-15)12-20(19)17-7-4-8-18(13-17)27-14-21(24)25/h1-10,12-13H,11,14H2,(H2,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534272

(CHEMBL4593409)Show SMILES C[C@H](Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H23NO3/c1-16(24(27)28)12-18-8-5-9-20(14-18)22-15-19(10-11-21(22)23(25)26)13-17-6-3-2-4-7-17/h2-11,14-16H,12-13H2,1H3,(H2,25,26)(H,27,28)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534278

(CHEMBL4533403)Show SMILES CCC(Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O Show InChI InChI=1S/C25H25NO3/c1-2-20(25(28)29)14-18-9-6-10-21(15-18)23-16-19(11-12-22(23)24(26)27)13-17-7-4-3-5-8-17/h3-12,15-16,20H,2,13-14H2,1H3,(H2,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534277

(CHEMBL4453126)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CC(C2CC2)C(O)=O)c1 Show InChI InChI=1S/C26H25NO3/c27-25(28)22-12-9-19(13-17-5-2-1-3-6-17)15-23(22)21-8-4-7-18(14-21)16-24(26(29)30)20-10-11-20/h1-9,12,14-15,20,24H,10-11,13,16H2,(H2,27,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534275

(CHEMBL4451830)Show SMILES CC(Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O Show InChI InChI=1S/C24H23NO3/c1-16(24(27)28)12-18-8-5-9-20(14-18)22-15-19(10-11-21(22)23(25)26)13-17-6-3-2-4-7-17/h2-11,14-16H,12-13H2,1H3,(H2,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534273

(CHEMBL4442238)Show InChI InChI=1S/C21H17NO3/c22-20(23)18-10-9-15(11-14-5-2-1-3-6-14)12-19(18)16-7-4-8-17(13-16)21(24)25/h1-10,12-13H,11H2,(H2,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534279

(CHEMBL4468927)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CC(O)=O)c1 Show InChI InChI=1S/C22H19NO3/c23-22(26)19-10-9-17(11-15-5-2-1-3-6-15)13-20(19)18-8-4-7-16(12-18)14-21(24)25/h1-10,12-13H,11,14H2,(H2,23,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human OATP1B1 expressed in HEK293 cells assessed as reduction in pivastatin uptake |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534281

(CHEMBL4536076)Show InChI InChI=1S/C14H13NO/c15-14(16)13-8-6-12(7-9-13)10-11-4-2-1-3-5-11/h1-9H,10H2,(H2,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534271

(CHEMBL4469134)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(OCC(O)=O)c1 Show InChI InChI=1S/C22H19NO4/c23-22(26)19-10-9-16(11-15-5-2-1-3-6-15)12-20(19)17-7-4-8-18(13-17)27-14-21(24)25/h1-10,12-13H,11,14H2,(H2,23,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534276

(CHEMBL4555962)Show SMILES C[C@@H](Cc1cccc(c1)-c1cc(Cc2ccccc2)ccc1C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H23NO3/c1-16(24(27)28)12-18-8-5-9-20(14-18)22-15-19(10-11-21(22)23(25)26)13-17-6-3-2-4-7-17/h2-11,14-16H,12-13H2,1H3,(H2,25,26)(H,27,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50534280

(CHEMBL4591759)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCCC(O)=O)c1 Show InChI InChI=1S/C24H23NO3/c25-24(28)21-13-12-19(14-17-6-2-1-3-7-17)16-22(21)20-10-4-8-18(15-20)9-5-11-23(26)27/h1-4,6-8,10,12-13,15-16H,5,9,11,14H2,(H2,25,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50534281

(CHEMBL4536076)Show InChI InChI=1S/C14H13NO/c15-14(16)13-8-6-12(7-9-13)10-11-4-2-1-3-5-11/h1-9H,10H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate preincubated for 10 mins foll... |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by patch clamp assay |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily D member 3
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human Kv4.3 expressed in CHO cells by patch clamp assay |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1H
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human Cav3.2 expressed in CHO cells by patch clamp assay |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 expressed in CHO cells by patch clamp assay |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50534274

(CHEMBL4457557)Show SMILES NC(=O)c1ccc(Cc2ccccc2)cc1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C23H21NO3/c24-23(27)20-11-9-18(13-16-5-2-1-3-6-16)15-21(20)19-8-4-7-17(14-19)10-12-22(25)26/h1-9,11,14-15H,10,12-13H2,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca
Curated by ChEMBL
| Assay Description
Inhibition of human Cav1.2 expressed in CHO cells by patch clamp assay |
ACS Med Chem Lett 7: 884-889 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00188
BindingDB Entry DOI: 10.7270/Q23N26W0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data