

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459018 (CHEMBL4166405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535970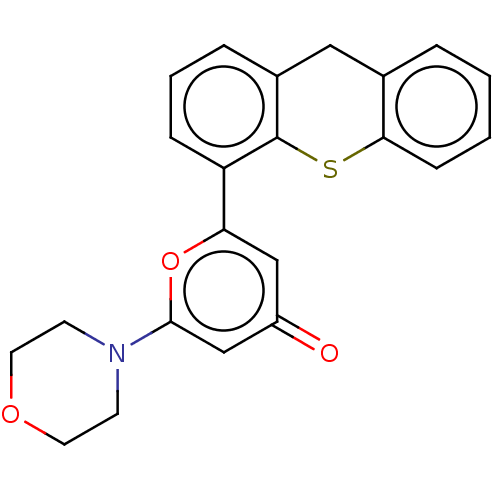 (CHEMBL4569967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459007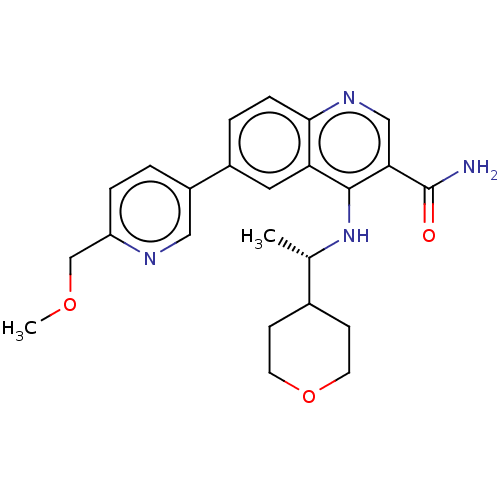 (CHEMBL4218734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535972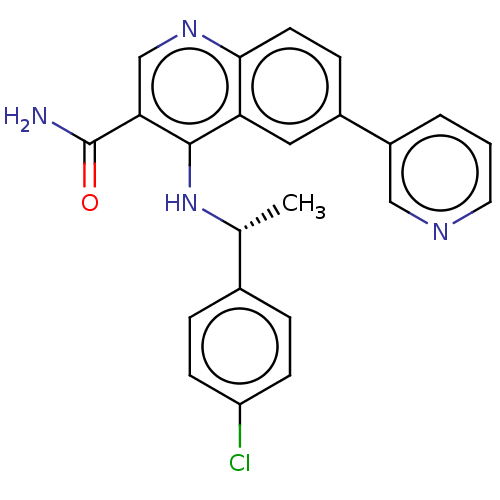 (CHEMBL4565988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50208517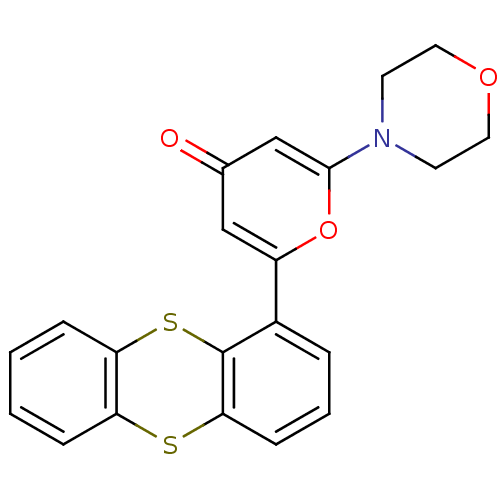 (2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50535972 (CHEMBL4565988) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DNAPK (unknown origin) using p53-based peptide substrate preincubated for 5 min prior to ATP addition | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535971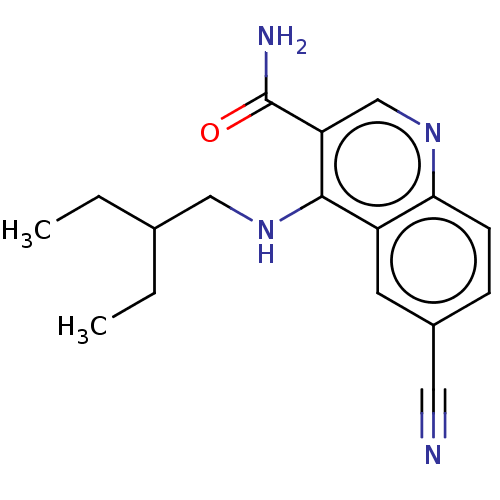 (CHEMBL4593348) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50535972 (CHEMBL4565988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kalpha using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459011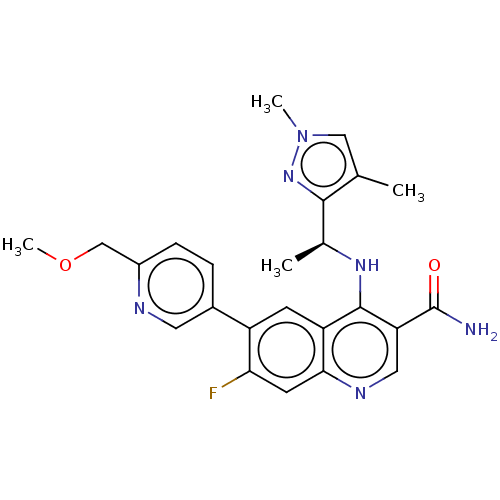 (CHEMBL4215266) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535995 (CHEMBL4517663) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50145038 (CHEMBL2143829) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50535972 (CHEMBL4565988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kbeta using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459018 (CHEMBL4166405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50535990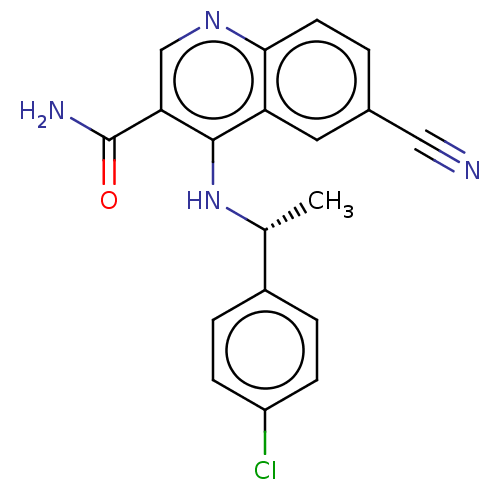 (CHEMBL4527822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kalpha using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50536001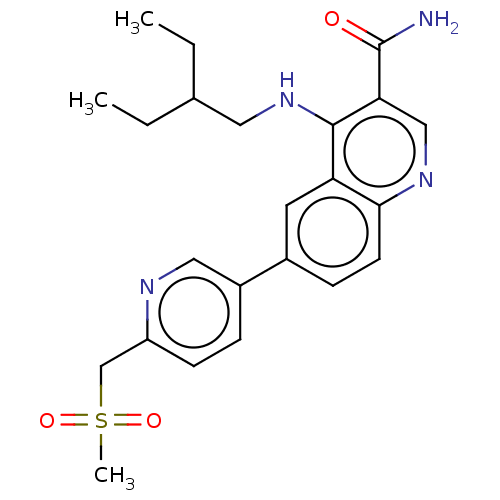 (CHEMBL4576302) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535966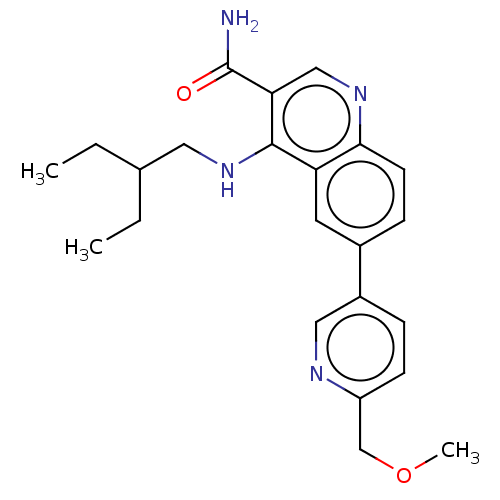 (CHEMBL4560522) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50535972 (CHEMBL4565988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kgamma using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459007 (CHEMBL4218734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535990 (CHEMBL4527822) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535982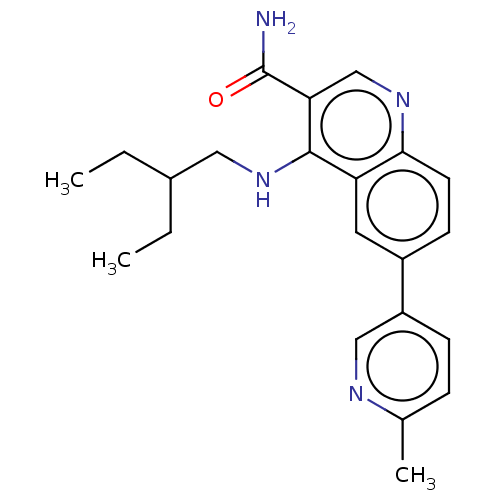 (CHEMBL4567042) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535979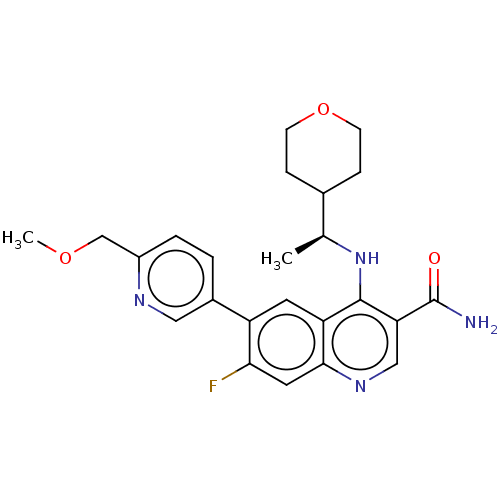 (CHEMBL4533539) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535994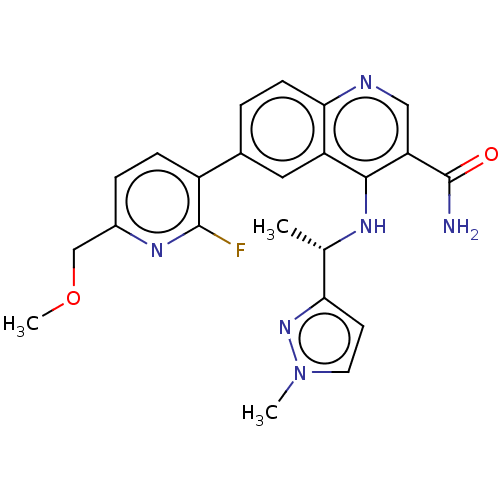 (CHEMBL4548927) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50536000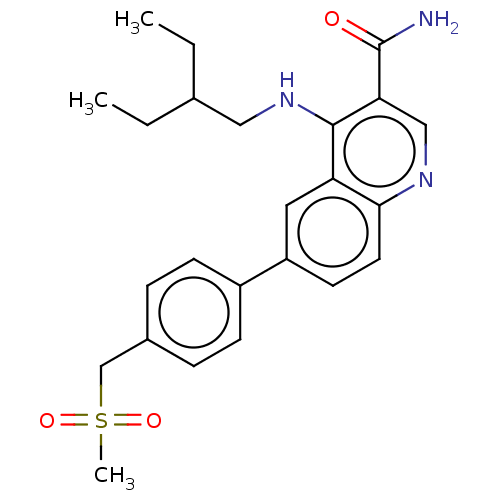 (CHEMBL4519670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535974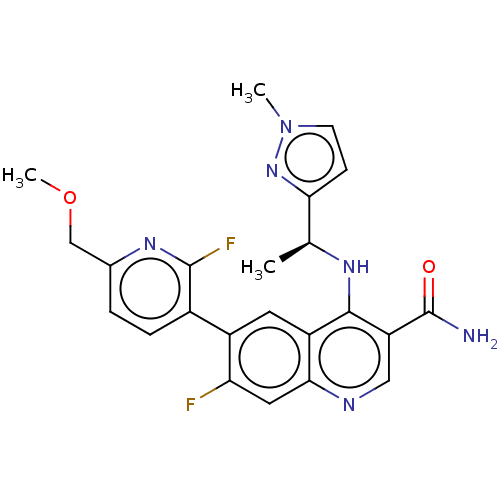 (CHEMBL4563993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535969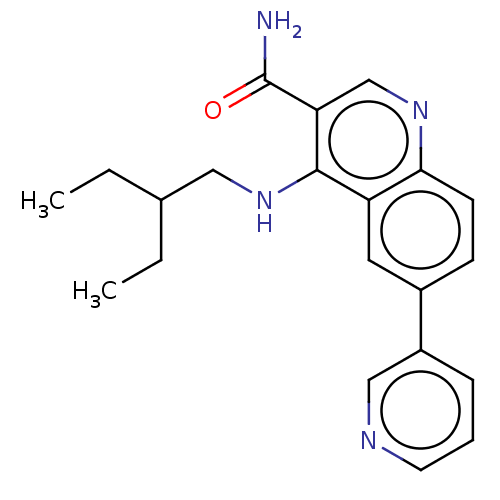 (CHEMBL4543897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535981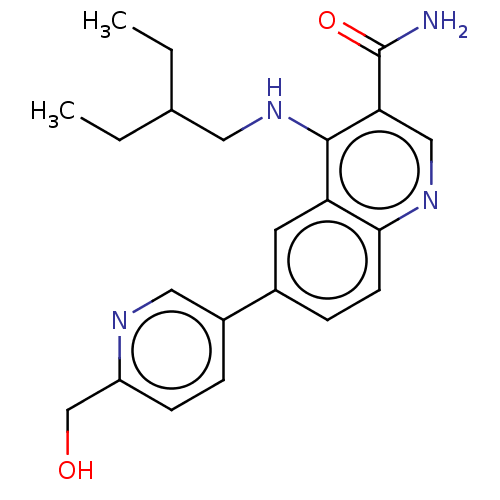 (CHEMBL4588822) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50535971 (CHEMBL4593348) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DNAPK (unknown origin) using p53-based peptide substrate preincubated for 5 min prior to ATP addition | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50535990 (CHEMBL4527822) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DNAPK (unknown origin) using p53-based peptide substrate preincubated for 5 min prior to ATP addition | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535972 (CHEMBL4565988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50536004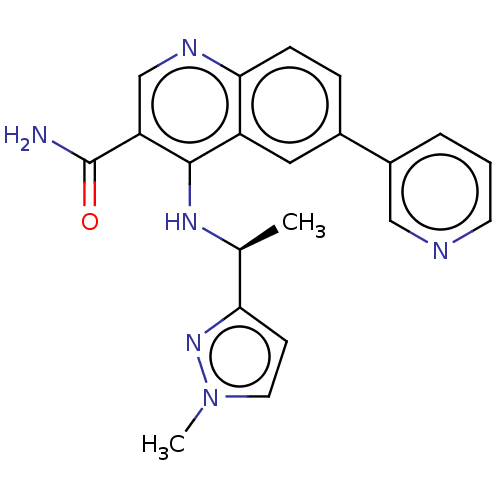 (CHEMBL4591401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535980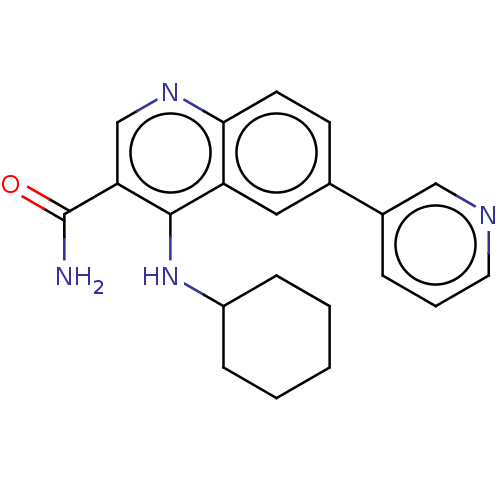 (CHEMBL4514524) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50208517 (2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kbeta using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535977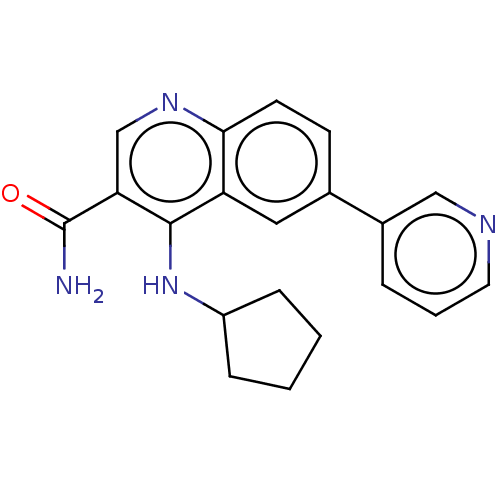 (CHEMBL4578468) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50535990 (CHEMBL4527822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kbeta using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535970 (CHEMBL4569967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535985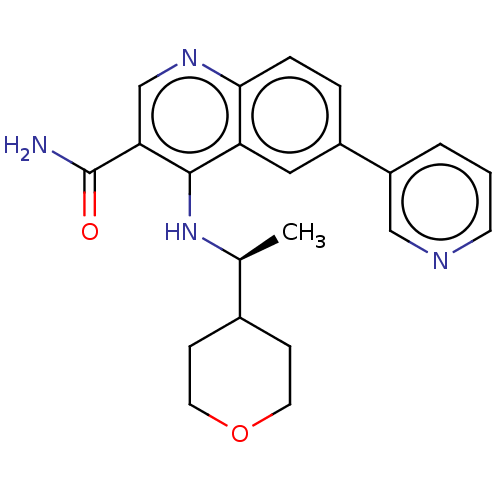 (CHEMBL4548199) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50535972 (CHEMBL4565988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of C-terminal FLAG-tagged recombinant mTOR (1362 to 2549 residues) (unknown origin) using Biotin-Ahx-Lys-Lys-Ala-Asn-Gln-Val-Phe-Leu-Gly-P... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50208517 (2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DNAPK (unknown origin) using p53-based peptide substrate preincubated for 5 min prior to ATP addition | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50535970 (CHEMBL4569967) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DNAPK (unknown origin) using p53-based peptide substrate preincubated for 5 min prior to ATP addition | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50535990 (CHEMBL4527822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kgamma using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50535971 (CHEMBL4593348) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kalpha using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50535991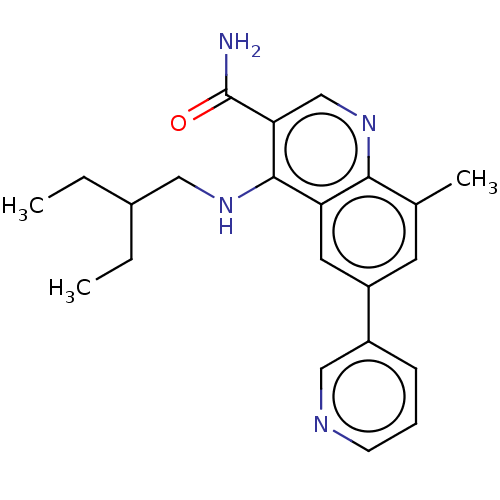 (CHEMBL4543599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50459018 (CHEMBL4166405) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DNAPK (unknown origin) using p53-based peptide substrate preincubated for 5 min prior to ATP addition | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535999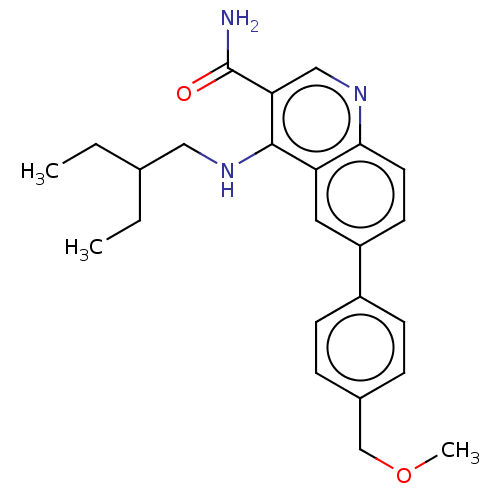 (CHEMBL4551177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535984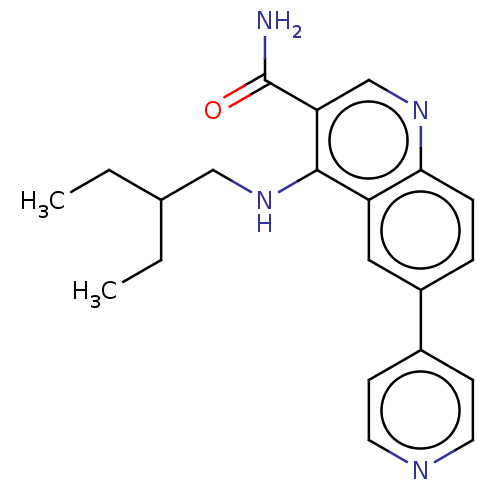 (CHEMBL4569880) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 418 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50459018 (CHEMBL4166405) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 445 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kalpha using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50535970 (CHEMBL4569967) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 452 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kalpha using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535998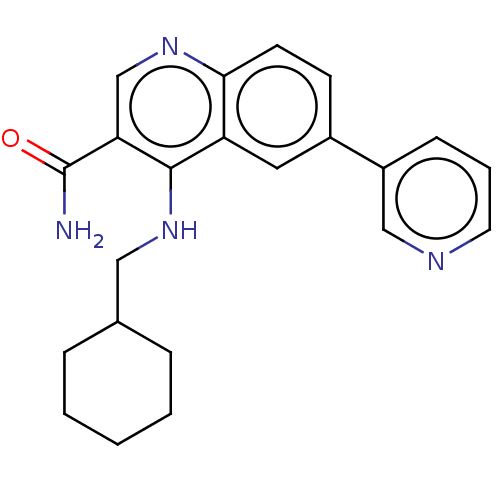 (CHEMBL4550293) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 456 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50208517 (2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 458 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kalpha using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535996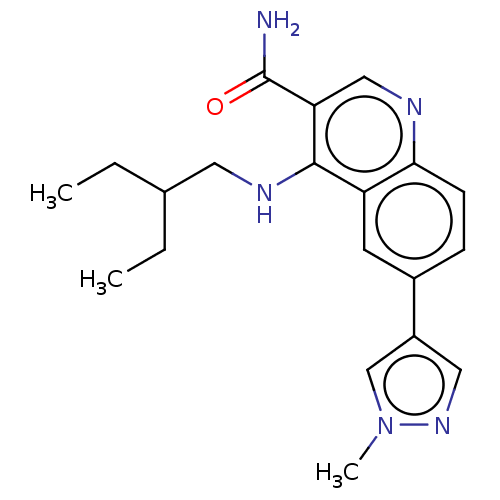 (CHEMBL4549865) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 525 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 191 total ) | Next | Last >> |