

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536604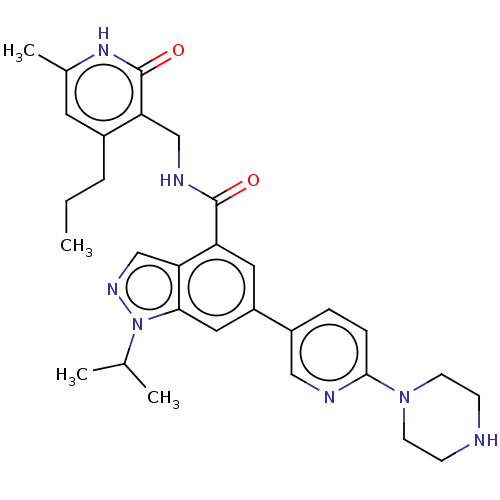 (CHEMBL4594174) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536589 (CHEMBL4545986) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536579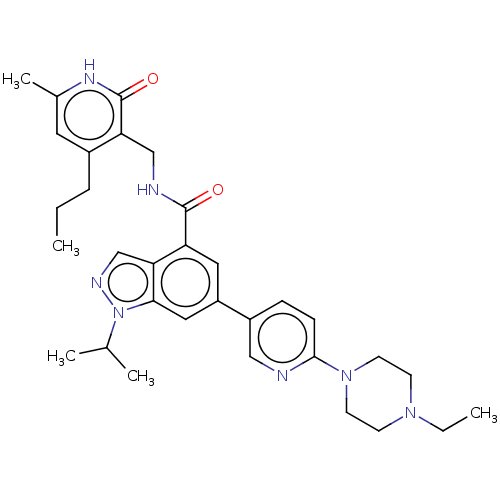 (CHEMBL4557033) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075071 (CHEMBL3414619) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536574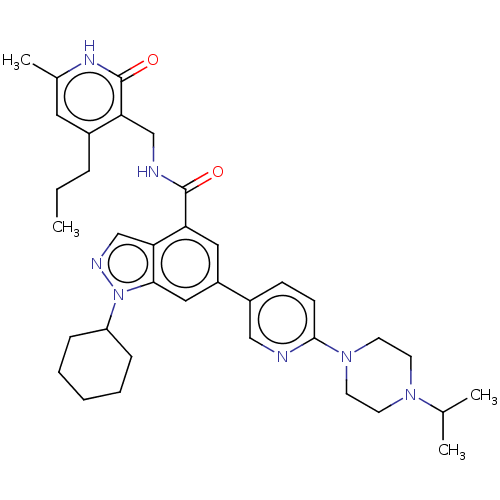 (CHEMBL4521710) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536573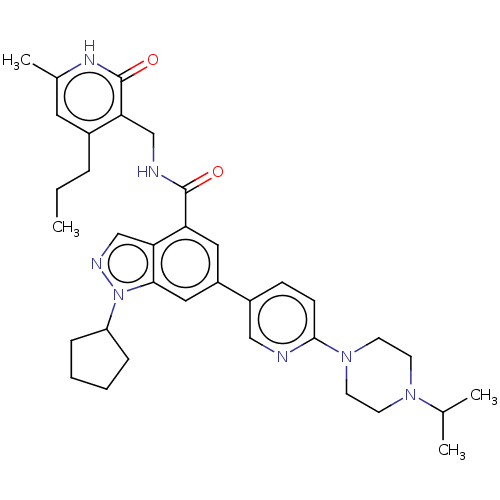 (CHEMBL4559875) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536572 (CHEMBL4539055) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536560 (CHEMBL4567859) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536563 (CHEMBL4559801) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536585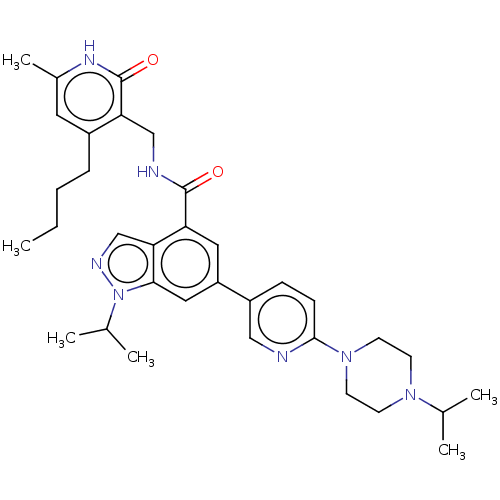 (CHEMBL4554286) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536598 (CHEMBL4551319) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536593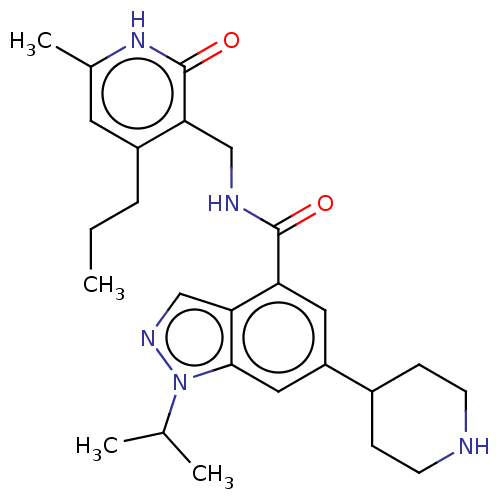 (CHEMBL4569399) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536584 (CHEMBL4544978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536555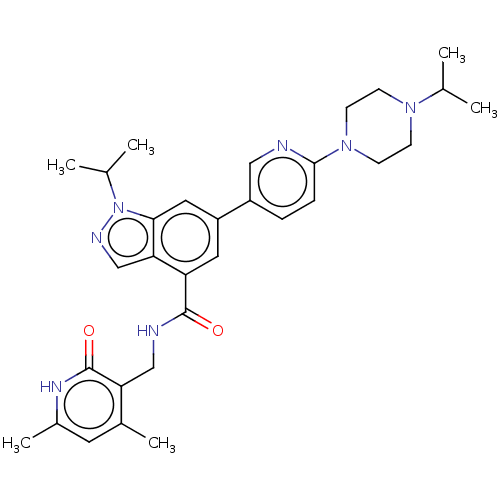 (CHEMBL4532174) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536582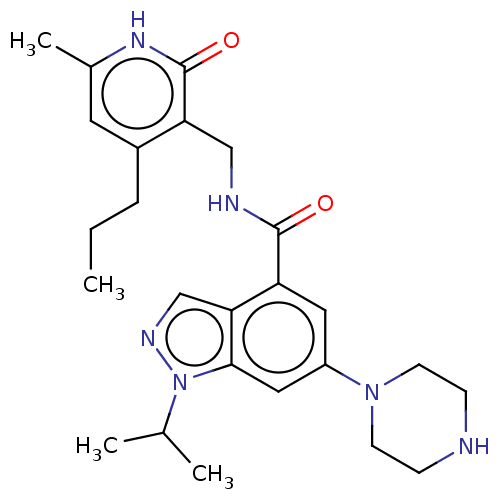 (CHEMBL4575460) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536581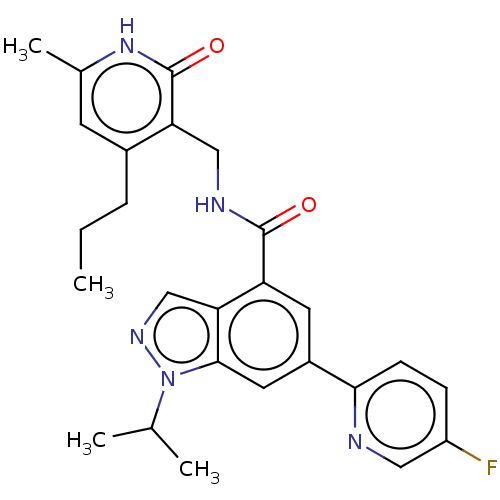 (CHEMBL4593352) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536562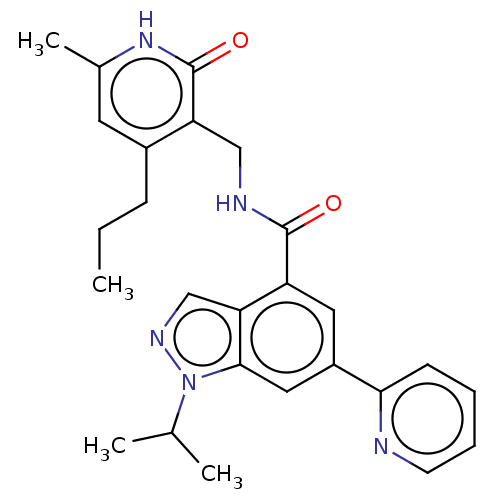 (CHEMBL4542135) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536578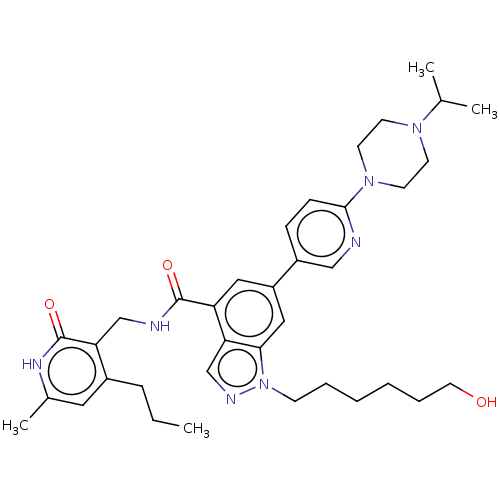 (CHEMBL4529085) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536595 (CHEMBL4535178) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536567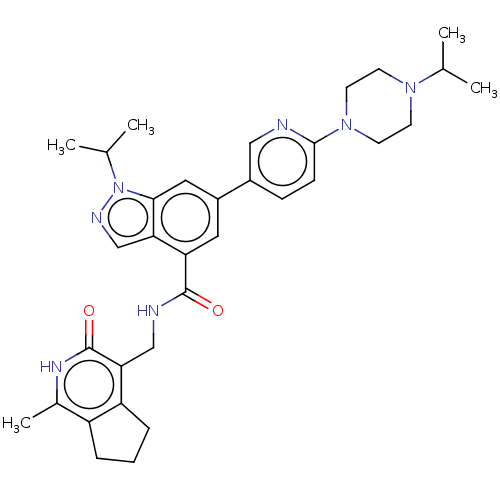 (CHEMBL4532360) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536596 (CHEMBL4534613) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536600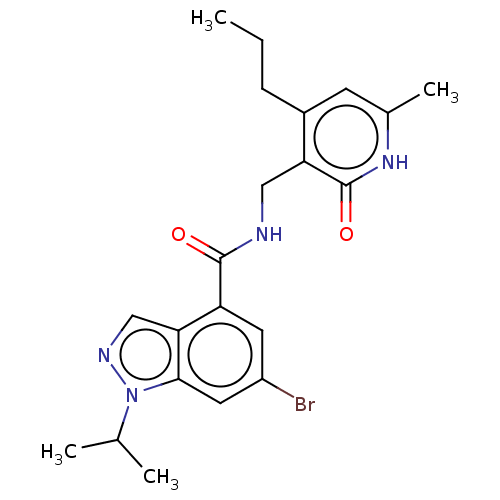 (CHEMBL4584071) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536554 (CHEMBL4535507) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536583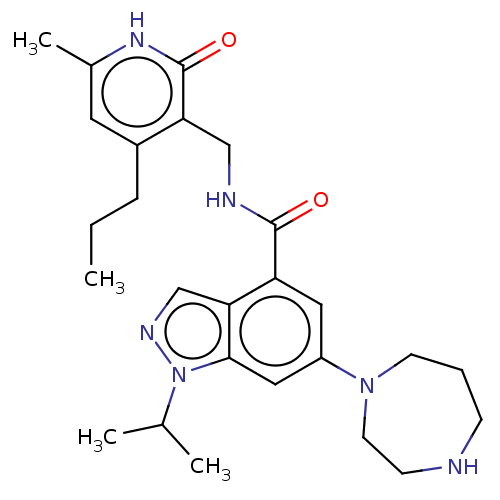 (CHEMBL4533229) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536592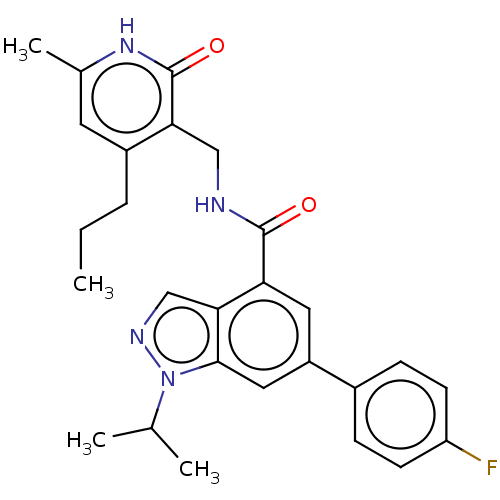 (CHEMBL4554500) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536575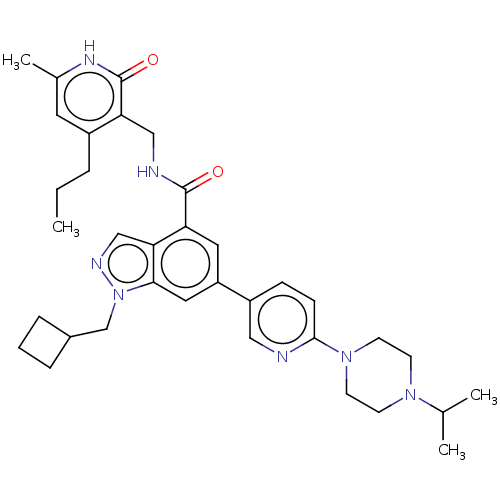 (CHEMBL4521620) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536604 (CHEMBL4594174) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536599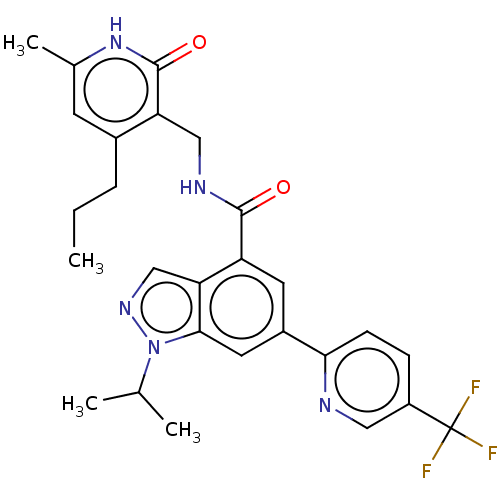 (CHEMBL4520484) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536580 (CHEMBL4566302) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50075071 (CHEMBL3414619) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536605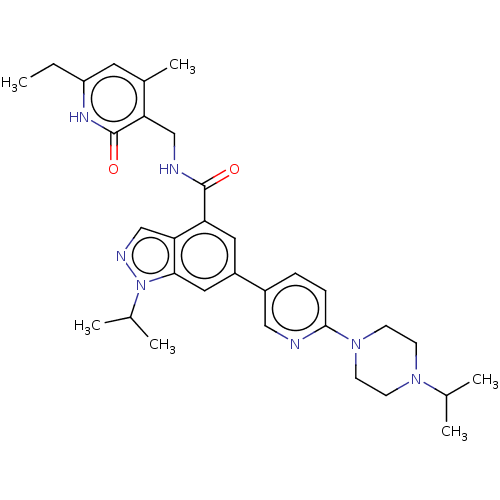 (CHEMBL4483642) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536560 (CHEMBL4567859) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536579 (CHEMBL4557033) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536573 (CHEMBL4559875) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536559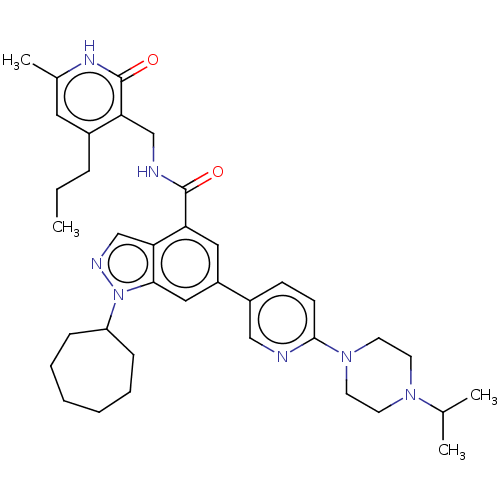 (CHEMBL4535050) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536589 (CHEMBL4545986) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536572 (CHEMBL4539055) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536557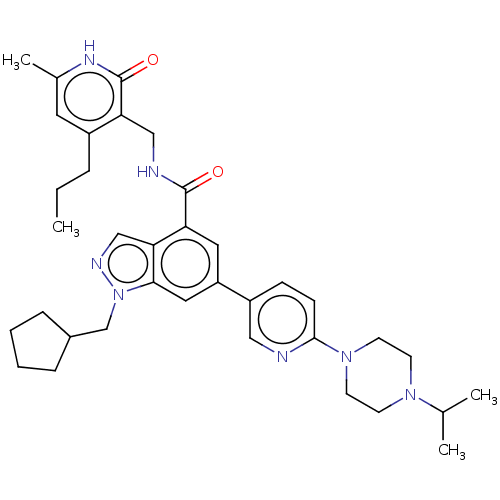 (CHEMBL4568471) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536585 (CHEMBL4554286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536590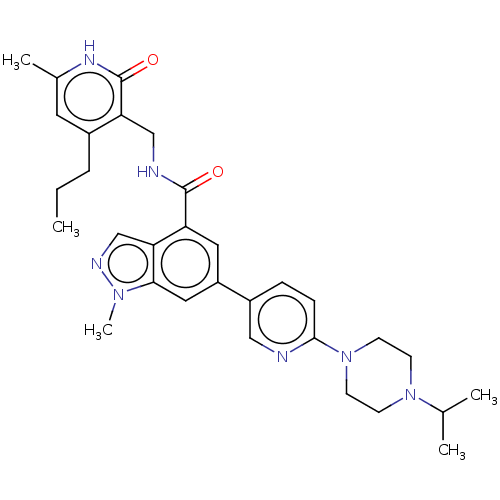 (CHEMBL4539350) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536598 (CHEMBL4551319) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536603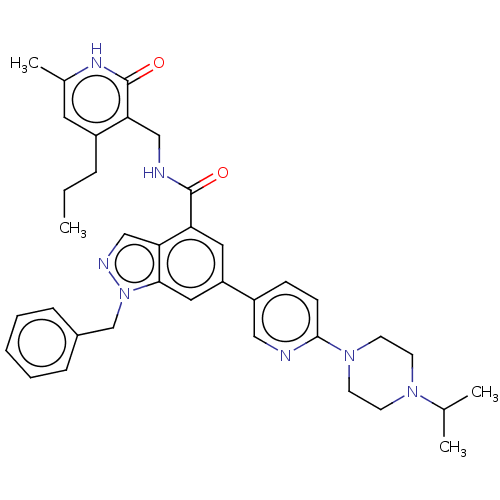 (CHEMBL4572342) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536574 (CHEMBL4521710) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536563 (CHEMBL4559801) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536553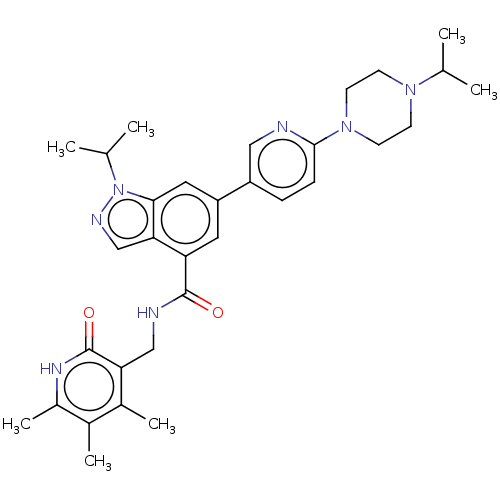 (CHEMBL4525296) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 328 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536597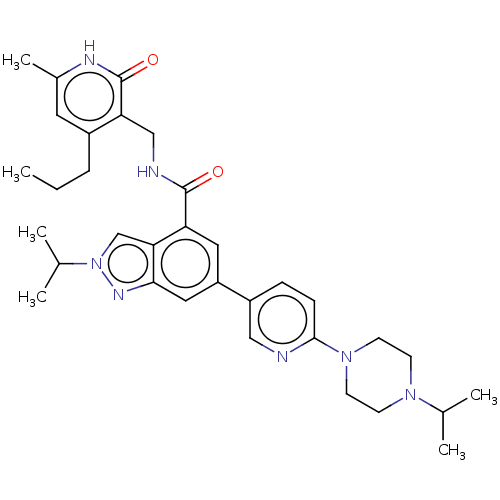 (CHEMBL4521009) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536578 (CHEMBL4529085) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50536558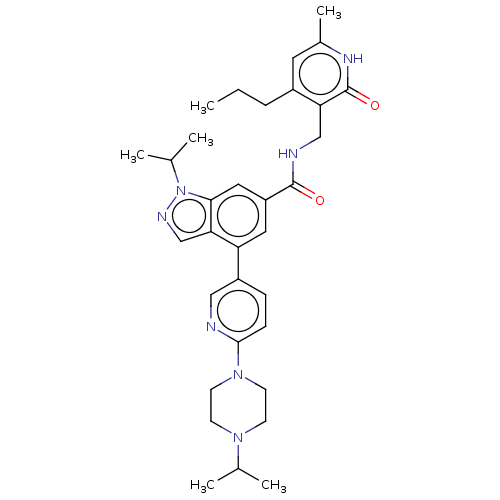 (CHEMBL4521826) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH2 histone methyltransferase activity of EZH2/EED/SUZ12 protein complex (unknown origin) using histone H3 peptide as substrate (21 to... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536593 (CHEMBL4569399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 613 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50536562 (CHEMBL4542135) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 681 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... | J Med Chem 59: 7617-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00855 BindingDB Entry DOI: 10.7270/Q2JW8JC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |