

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562160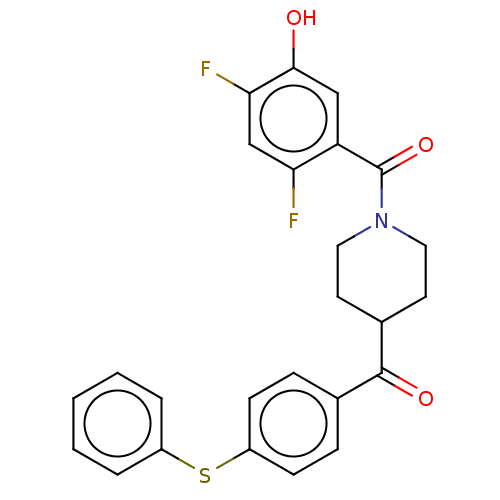 (CHEMBL4757403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562158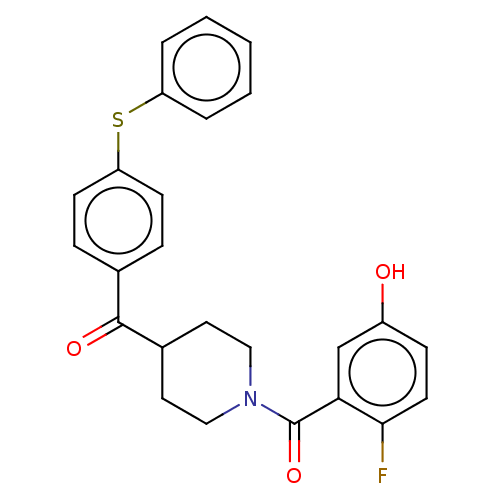 (CHEMBL4747585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562161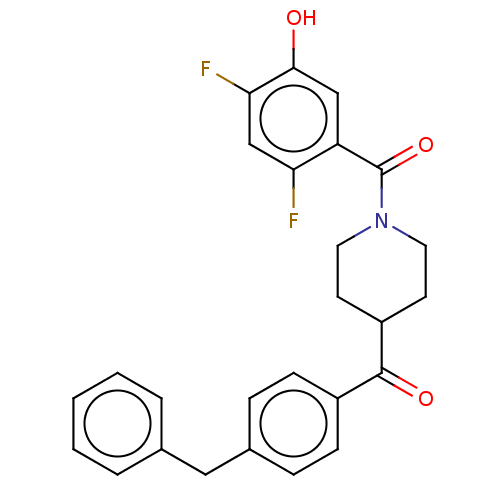 (CHEMBL4755606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562159 (CHEMBL4798508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562160 (CHEMBL4757403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562158 (CHEMBL4747585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562138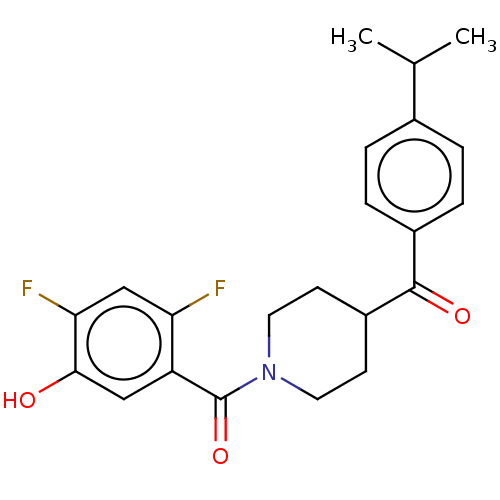 (CHEMBL4758717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562161 (CHEMBL4755606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562159 (CHEMBL4798508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562151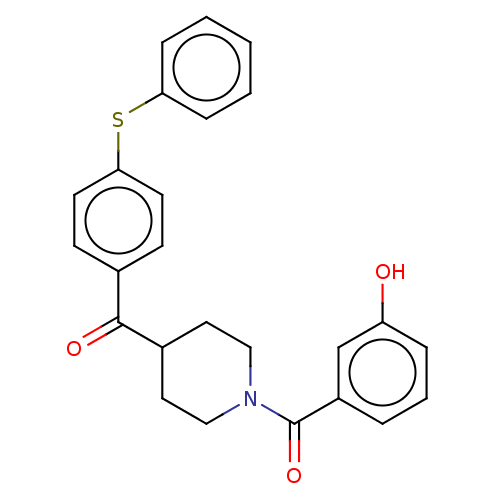 (CHEMBL4789288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562154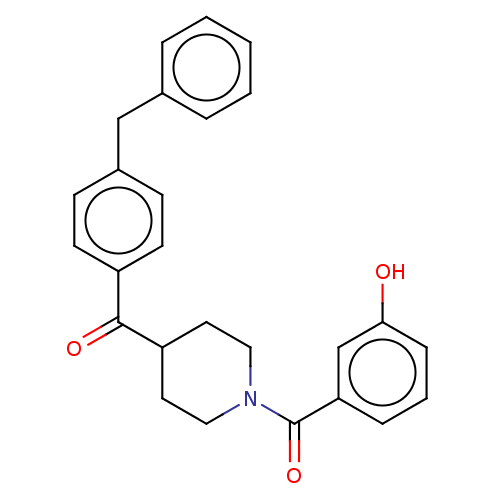 (CHEMBL4758976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50531972 (CHEMBL4536045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562147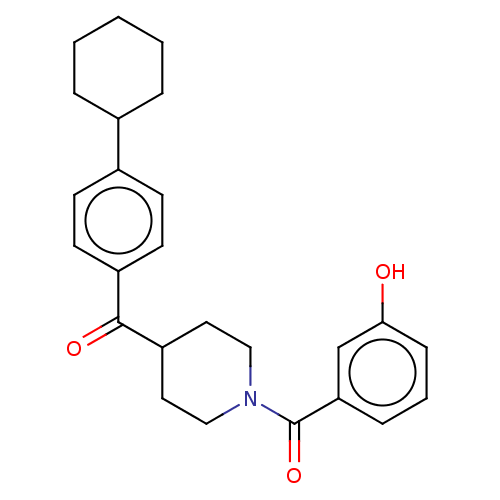 (CHEMBL4798272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414921 (CHEMBL570812) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50531982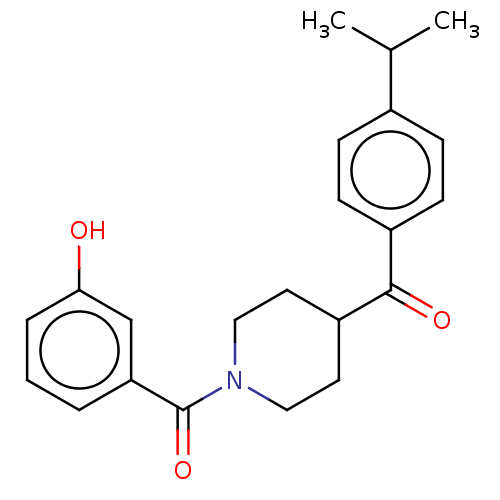 (CHEMBL4435479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562150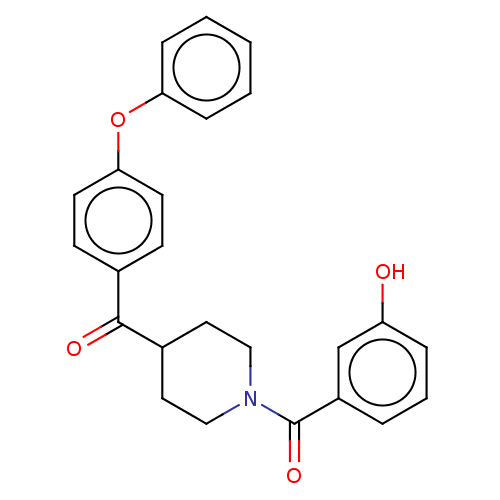 (CHEMBL4746545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562148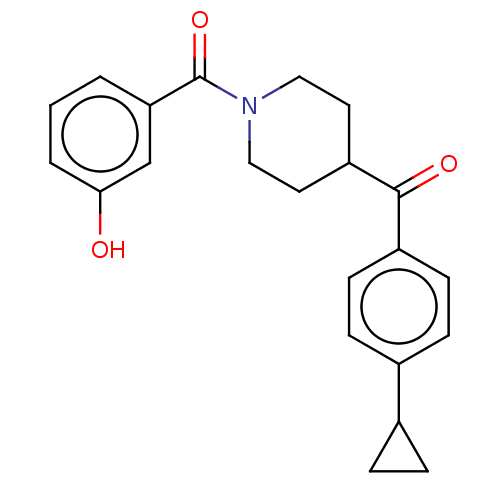 (CHEMBL4794653) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562160 (CHEMBL4757403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 568 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of MAGL in human U937 cells assessed as reduction in [3H]glycerol formation using [1,2,3-3H]2-OG as substrate incubated for 15 mins by liq... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562149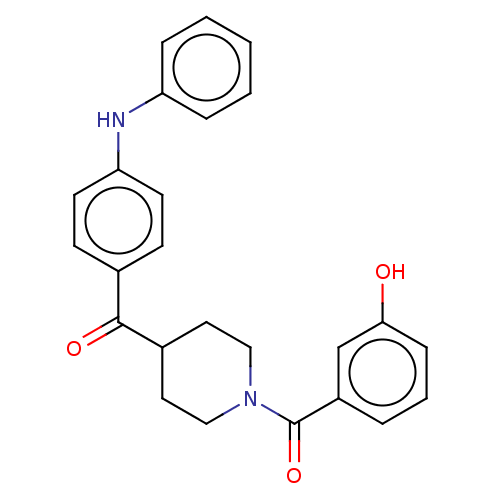 (CHEMBL4753531) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 758 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562159 (CHEMBL4798508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 844 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of MAGL in human U937 cells assessed as reduction in [3H]glycerol formation using [1,2,3-3H]2-OG as substrate incubated for 15 mins by liq... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562158 (CHEMBL4747585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 868 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of MAGL in human U937 cells assessed as reduction in [3H]glycerol formation using [1,2,3-3H]2-OG as substrate incubated for 15 mins by liq... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562155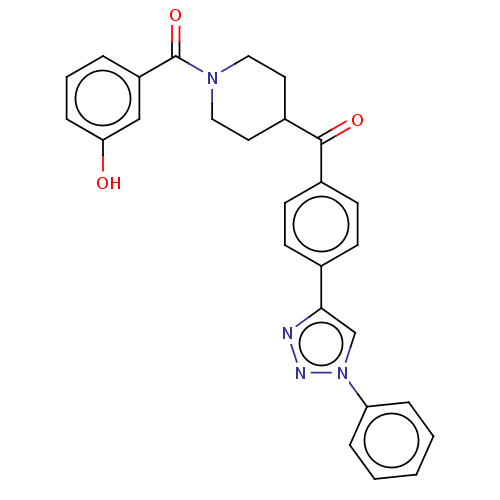 (CHEMBL4784401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562161 (CHEMBL4755606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of MAGL in human U937 cells assessed as reduction in [3H]glycerol formation using [1,2,3-3H]2-OG as substrate incubated for 15 mins by liq... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562144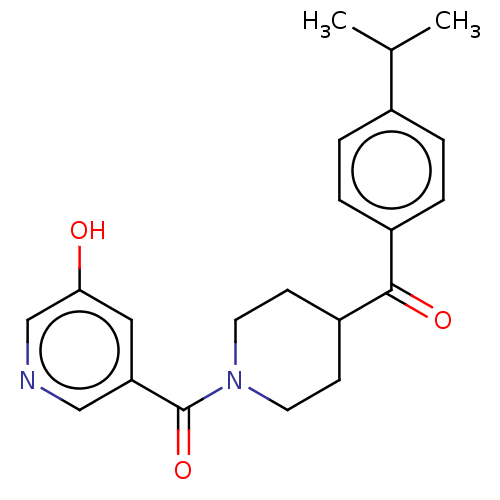 (CHEMBL4753368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562142 (CHEMBL4754790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562157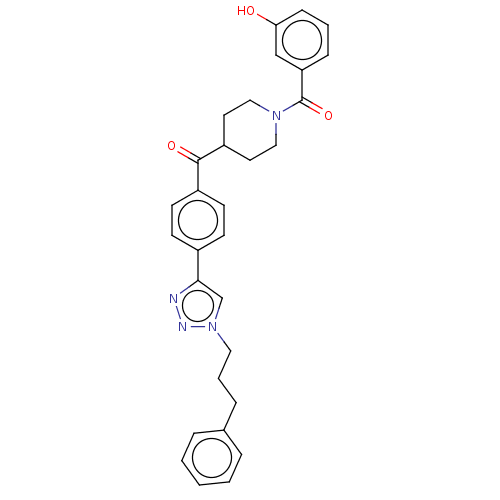 (CHEMBL4784941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562152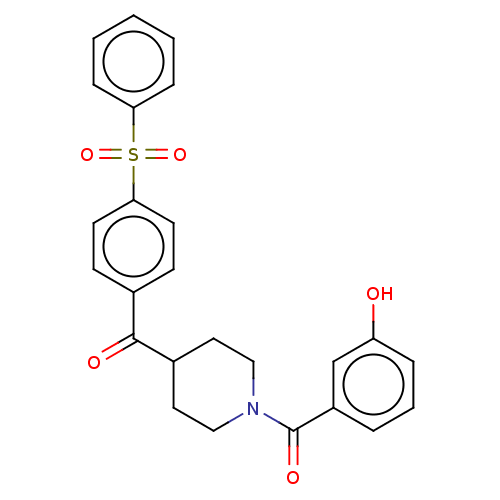 (CHEMBL4755379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562153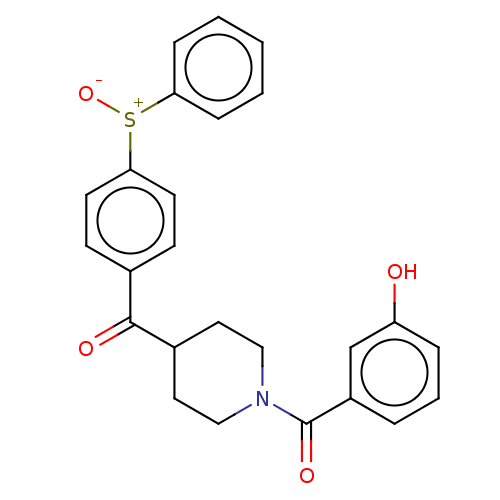 (CHEMBL4763206) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562156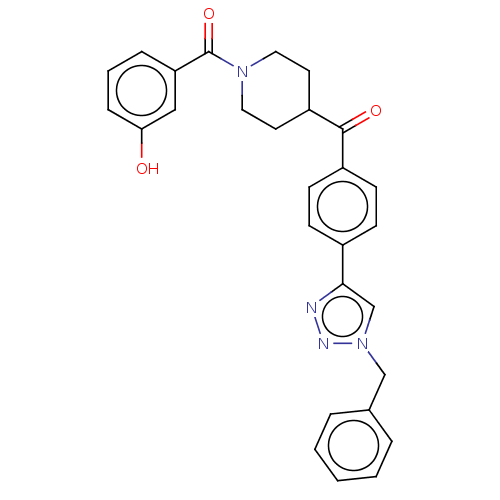 (CHEMBL4758281) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562140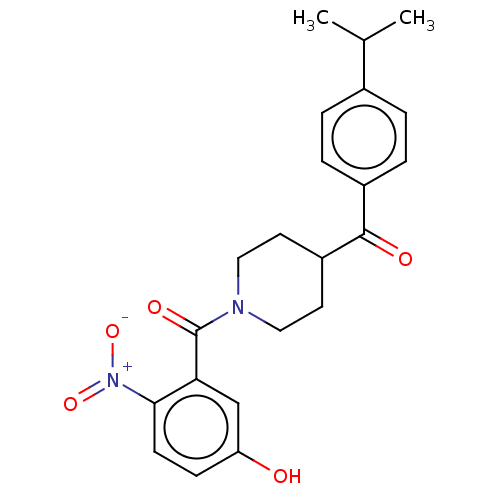 (CHEMBL4752739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562139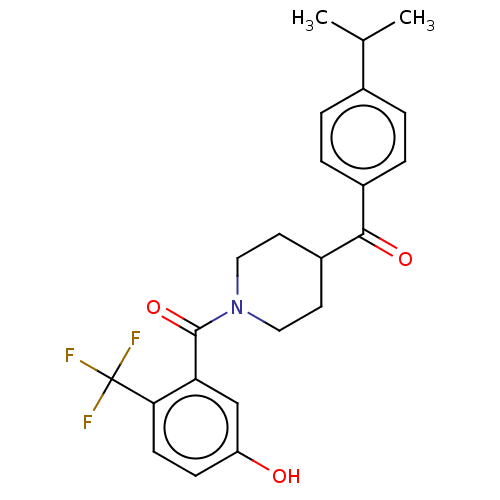 (CHEMBL4755839) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidylserine lipase ABHD12 (Homo sapiens (Human)) | BDBM50562161 (CHEMBL4755606) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human ABHD12 expressed in HEK293 cells using [3H]2-OG as substrate preincubated for 30 mins followed by substrate addition and measured... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50562158 (CHEMBL4747585) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of FAAH in human U937 cells assessed as reduction in [3H]ethanolamine formation using [ethanolamine-1-3H]AEA as substrate incubated for 15... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50562159 (CHEMBL4798508) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of FAAH in human U937 cells assessed as reduction in [3H]ethanolamine formation using [ethanolamine-1-3H]AEA as substrate incubated for 15... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50562160 (CHEMBL4757403) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of FAAH in human U937 cells assessed as reduction in [3H]ethanolamine formation using [ethanolamine-1-3H]AEA as substrate incubated for 15... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50562161 (CHEMBL4755606) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of FAAH in human U937 cells assessed as reduction in [3H]ethanolamine formation using [ethanolamine-1-3H]AEA as substrate incubated for 15... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562141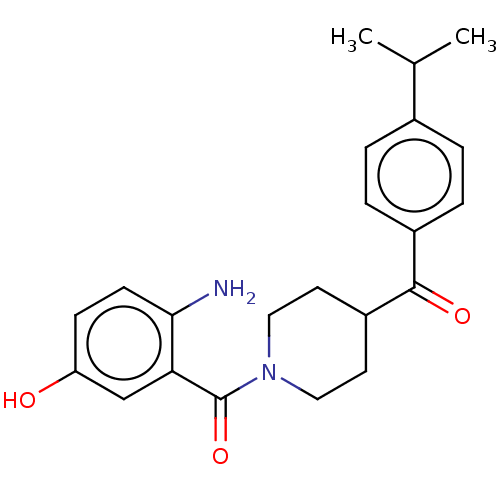 (CHEMBL4757544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAGL using 4-nitrophenylacetate as substrate incubated for 30 mins by microplate reader assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50562158 (CHEMBL4747585) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor incubated for 90 mins by radioligand binding assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50562159 (CHEMBL4798508) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor incubated for 90 mins by radioligand binding assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50562160 (CHEMBL4757403) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor incubated for 90 mins by radioligand binding assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50562161 (CHEMBL4755606) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor incubated for 90 mins by radioligand binding assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50562158 (CHEMBL4747585) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor incubated for 90 mins by radioligand binding assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50562159 (CHEMBL4798508) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor incubated for 90 mins by radioligand binding assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50562160 (CHEMBL4757403) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor incubated for 90 mins by radioligand binding assay | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidylserine lipase ABHD12 (Homo sapiens (Human)) | BDBM50562159 (CHEMBL4798508) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human ABHD12 expressed in HEK293 cells using [3H]2-OG as substrate preincubated for 30 mins followed by substrate addition and measured... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidylserine lipase ABHD12 (Homo sapiens (Human)) | BDBM50562158 (CHEMBL4747585) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human ABHD12 expressed in HEK293 cells using [3H]2-OG as substrate preincubated for 30 mins followed by substrate addition and measured... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Homo sapiens (Human)) | BDBM50562161 (CHEMBL4755606) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human ABHD6 expressed in HEK293 cells using [3H]2-OG as substrate preincubated for 30 mins followed by substrate addition and measured ... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Homo sapiens (Human)) | BDBM50562160 (CHEMBL4757403) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human ABHD6 expressed in HEK293 cells using [3H]2-OG as substrate preincubated for 30 mins followed by substrate addition and measured ... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Homo sapiens (Human)) | BDBM50562159 (CHEMBL4798508) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human ABHD6 expressed in HEK293 cells using [3H]2-OG as substrate preincubated for 30 mins followed by substrate addition and measured ... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Homo sapiens (Human)) | BDBM50562158 (CHEMBL4747585) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human ABHD6 expressed in HEK293 cells using [3H]2-OG as substrate preincubated for 30 mins followed by substrate addition and measured ... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |