 Found 50 hits Enz. Inhib. hit(s) with all data for entry = 50013492
Found 50 hits Enz. Inhib. hit(s) with all data for entry = 50013492 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566635
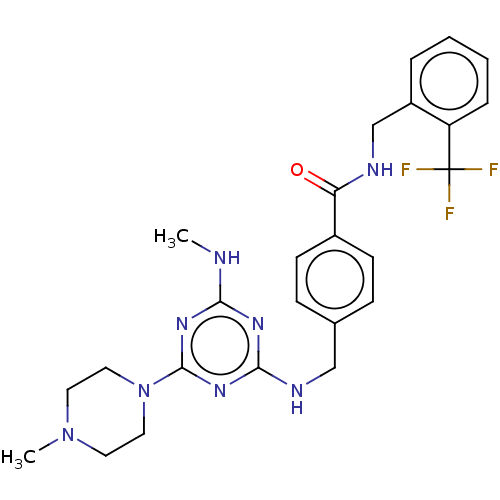
(CHEMBL4875337)Show SMILES CNc1nc(NCc2ccc(cc2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566638
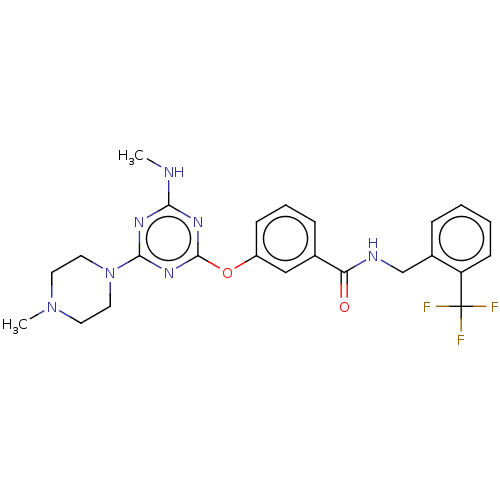
(CHEMBL4862566)Show SMILES CNc1nc(Oc2cccc(c2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566636

(CHEMBL4870025)Show SMILES CNc1nc(NCc2cc(oc2C)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50539700
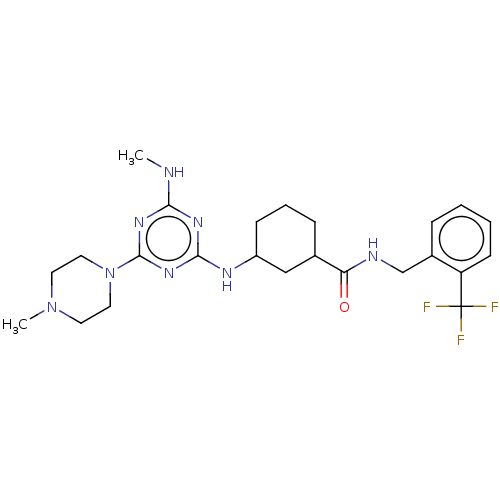
(CHEMBL4637413)Show SMILES CNc1nc(NC2CCCC(C2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 Show InChI InChI=1S/C24H33F3N8O/c1-28-21-31-22(33-23(32-21)35-12-10-34(2)11-13-35)30-18-8-5-7-16(14-18)20(36)29-15-17-6-3-4-9-19(17)24(25,26)27/h3-4,6,9,16,18H,5,7-8,10-15H2,1-2H3,(H,29,36)(H2,28,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566637
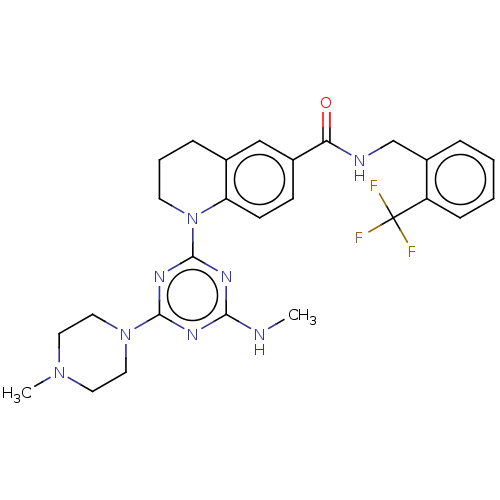
(CHEMBL4871885)Show SMILES CNc1nc(nc(n1)N1CCCc2cc(ccc12)C(=O)NCc1ccccc1C(F)(F)F)N1CCN(C)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566632
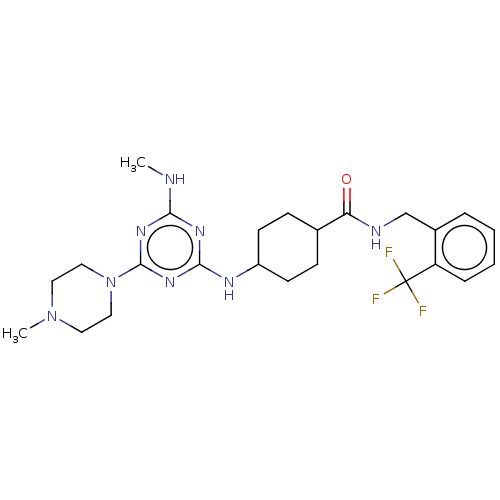
(CHEMBL4850549)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 |(45.76,-44.82,;44.43,-45.6,;44.44,-47.14,;43.11,-47.91,;43.12,-49.45,;41.78,-50.22,;40.45,-49.45,;39.11,-50.22,;37.79,-49.45,;37.78,-47.91,;39.11,-47.13,;40.45,-47.91,;36.45,-47.15,;36.44,-45.61,;35.12,-47.92,;33.78,-47.16,;32.45,-47.93,;31.11,-47.17,;29.79,-47.94,;29.78,-49.49,;31.12,-50.26,;32.46,-49.48,;33.79,-50.25,;34.55,-48.91,;35.33,-50.24,;33.79,-51.79,;44.45,-50.22,;45.78,-49.45,;45.78,-47.91,;47.11,-50.22,;47.1,-51.76,;48.43,-52.53,;49.77,-51.77,;51.1,-52.55,;49.77,-50.23,;48.44,-49.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435763
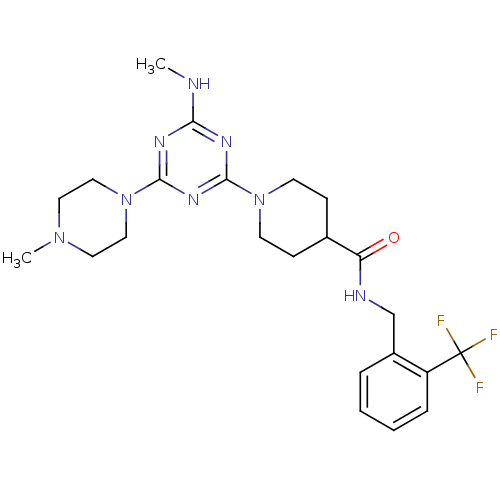
(CHEMBL2392711)Show SMILES CNc1nc(nc(n1)N1CCN(C)CC1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C23H31F3N8O/c1-27-20-29-21(31-22(30-20)34-13-11-32(2)12-14-34)33-9-7-16(8-10-33)19(35)28-15-17-5-3-4-6-18(17)23(24,25)26/h3-6,16H,7-15H2,1-2H3,(H,28,35)(H,27,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566631
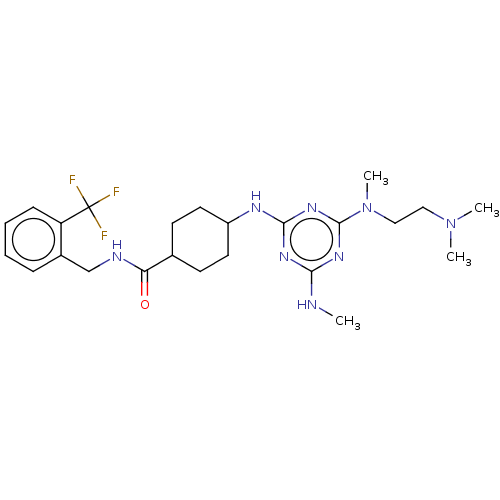
(CHEMBL4852295)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N(C)CCN(C)C |(19.35,-45.17,;18.02,-45.94,;18.02,-47.48,;16.7,-48.25,;16.7,-49.79,;15.37,-50.56,;14.04,-49.79,;12.7,-50.56,;11.37,-49.79,;11.37,-48.26,;12.7,-47.47,;14.03,-48.25,;10.03,-47.49,;10.03,-45.95,;8.7,-48.27,;7.37,-47.5,;6.04,-48.28,;4.7,-47.51,;3.37,-48.28,;3.37,-49.83,;4.7,-50.6,;6.04,-49.83,;7.37,-50.59,;8.14,-49.26,;8.91,-50.58,;7.38,-52.13,;18.03,-50.56,;19.37,-49.79,;19.37,-48.25,;20.7,-50.56,;20.7,-52.1,;22.03,-49.79,;23.37,-50.56,;24.7,-49.79,;26.03,-50.57,;24.7,-48.25,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566629
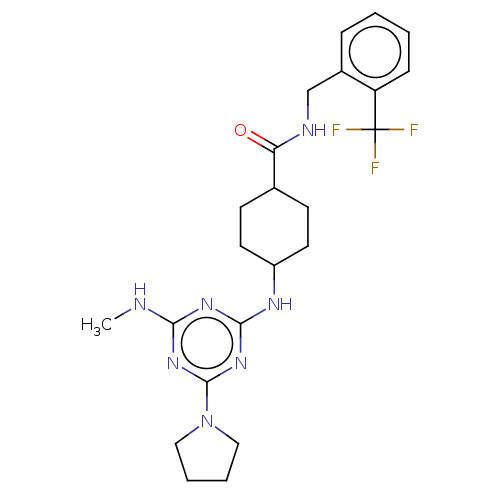
(CHEMBL4846972)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCCC1 |(45.64,-30.95,;44.31,-31.72,;44.31,-33.26,;42.98,-34.04,;42.99,-35.57,;41.66,-36.34,;40.33,-35.57,;38.99,-36.34,;37.66,-35.58,;37.66,-34.04,;38.99,-33.26,;40.32,-34.03,;36.32,-33.27,;36.32,-31.73,;34.99,-34.05,;33.66,-33.28,;32.33,-34.06,;30.99,-33.3,;29.66,-34.07,;29.66,-35.61,;30.99,-36.38,;32.33,-35.61,;33.66,-36.38,;34.42,-35.04,;35.2,-36.37,;33.67,-37.92,;44.32,-36.34,;45.65,-35.57,;45.66,-34.03,;46.99,-36.35,;47.15,-37.87,;48.65,-38.19,;49.43,-36.86,;48.4,-35.72,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566620
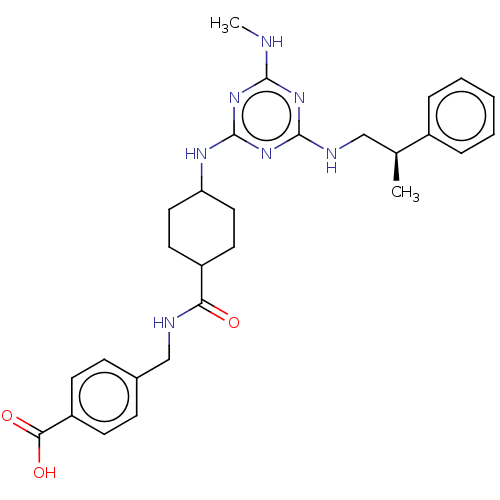
(CHEMBL4850535)Show SMILES CNc1nc(NC[C@H](C)c2ccccc2)nc(NC2CCC(CC2)C(=O)NCc2ccc(cc2)C(O)=O)n1 |r,wU:7.7,(21.46,-36.35,;20.13,-37.12,;20.13,-38.66,;21.47,-39.43,;21.47,-40.97,;22.81,-41.74,;24.14,-40.97,;25.47,-41.75,;25.47,-43.29,;26.81,-40.98,;28.14,-41.75,;29.47,-40.98,;29.47,-39.44,;28.13,-38.67,;26.8,-39.44,;20.14,-41.74,;18.81,-40.97,;17.48,-41.74,;16.14,-40.97,;14.81,-41.74,;13.48,-40.97,;13.48,-39.44,;14.81,-38.66,;16.14,-39.43,;12.14,-38.67,;12.14,-37.13,;10.81,-39.45,;9.48,-38.68,;8.14,-39.46,;8.15,-41.01,;6.81,-41.78,;5.48,-41.01,;5.48,-39.46,;6.81,-38.69,;4.14,-41.78,;2.81,-41.01,;4.14,-43.32,;18.8,-39.43,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566617
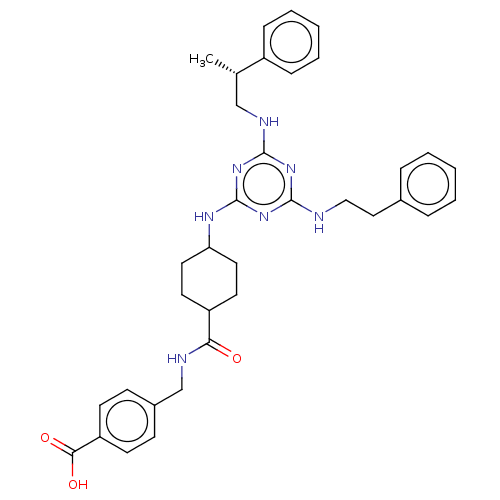
(CHEMBL4851061)Show SMILES C[C@@H](CNc1nc(NCCc2ccccc2)nc(NC2CCC(CC2)C(=O)NCc2ccc(cc2)C(O)=O)n1)c1ccccc1 |r,wU:1.0,(25.02,-12.86,;25.02,-11.32,;23.68,-10.55,;22.35,-11.32,;21.02,-10.55,;21.02,-9,;19.67,-8.24,;19.67,-6.7,;21,-5.92,;20.99,-4.38,;22.32,-3.61,;23.66,-4.38,;24.99,-3.61,;24.99,-2.07,;23.64,-1.3,;22.32,-2.08,;18.35,-9.01,;18.35,-10.54,;17.02,-11.31,;15.69,-10.54,;14.35,-11.31,;13.02,-10.55,;13.02,-9.01,;14.35,-8.23,;15.68,-9,;11.68,-8.25,;11.68,-6.71,;10.35,-9.02,;9.02,-8.26,;7.69,-9.03,;7.69,-10.58,;6.35,-11.35,;5.02,-10.58,;5.02,-9.04,;6.35,-8.27,;3.69,-11.35,;2.35,-10.58,;3.68,-12.89,;19.68,-11.32,;26.35,-10.55,;27.68,-11.32,;29.01,-10.56,;29.02,-9.01,;27.67,-8.24,;26.34,-9.02,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566618
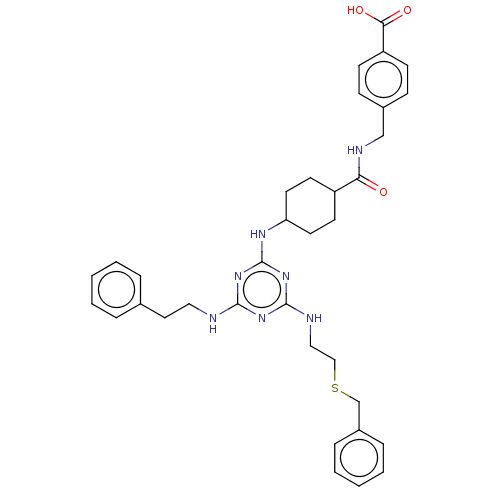
(CHEMBL4868985)Show SMILES OC(=O)c1ccc(CNC(=O)C2CCC(CC2)Nc2nc(NCCSCc3ccccc3)nc(NCCc3ccccc3)n2)cc1 |(35.66,-10.93,;36.99,-11.7,;36.99,-13.24,;38.32,-10.93,;39.66,-11.7,;41,-10.93,;40.99,-9.38,;42.32,-8.61,;43.66,-9.37,;44.99,-8.6,;44.98,-7.06,;46.33,-9.36,;46.33,-10.9,;47.66,-11.66,;48.99,-10.89,;48.99,-9.35,;47.65,-8.58,;50.32,-11.66,;51.66,-10.89,;52.99,-11.67,;54.32,-10.9,;55.65,-11.67,;56.99,-10.9,;58.32,-11.67,;59.65,-10.9,;60.99,-11.67,;62.32,-10.9,;63.65,-11.67,;64.98,-10.91,;64.99,-9.36,;63.64,-8.59,;62.32,-9.37,;54.32,-9.36,;52.98,-8.59,;52.97,-7.05,;54.3,-6.27,;54.3,-4.73,;55.63,-3.96,;56.97,-4.73,;58.3,-3.96,;58.29,-2.41,;56.95,-1.65,;55.62,-2.43,;51.65,-9.36,;39.65,-8.62,;38.33,-9.39,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566623

(CHEMBL4853666)Show SMILES CNc1nc(NC[C@H](C)c2ccccc2)nc(NC2CCC(CC2)C(=O)NCc2ccccc2OC(F)(F)F)n1 |r,wU:7.7,(55.34,-51.68,;54.01,-52.45,;54.01,-53.99,;55.36,-54.76,;55.35,-56.3,;56.69,-57.07,;58.02,-56.31,;59.36,-57.08,;59.35,-58.62,;60.69,-56.31,;62.02,-57.08,;63.35,-56.31,;63.35,-54.77,;62.01,-54,;60.68,-54.77,;54.02,-57.07,;52.69,-56.3,;51.36,-57.07,;50.03,-56.3,;48.69,-57.07,;47.36,-56.31,;47.36,-54.77,;48.69,-53.99,;50.02,-54.76,;46.02,-54,;46.02,-52.46,;44.69,-54.78,;43.36,-54.01,;42.03,-54.79,;40.69,-54.02,;39.36,-54.79,;39.36,-56.34,;40.69,-57.11,;42.03,-56.34,;43.37,-57.11,;43.36,-58.64,;42.58,-59.97,;41.82,-58.64,;44.7,-59.41,;52.68,-54.77,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566628
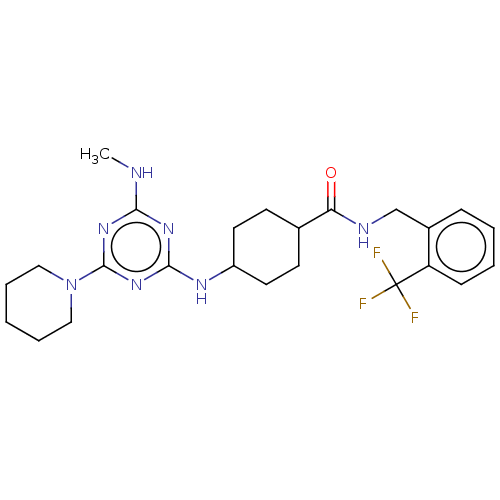
(CHEMBL4878345)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCCCC1 |(17.73,-31.09,;16.4,-31.87,;16.4,-33.41,;15.08,-34.18,;15.09,-35.72,;13.75,-36.49,;12.42,-35.72,;11.08,-36.49,;9.75,-35.72,;9.75,-34.18,;11.08,-33.4,;12.42,-34.18,;8.42,-33.42,;8.41,-31.88,;7.09,-34.19,;5.75,-33.43,;4.42,-34.2,;3.08,-33.44,;1.75,-34.21,;1.75,-35.76,;3.09,-36.53,;4.42,-35.75,;5.75,-36.52,;6.52,-35.18,;7.29,-36.51,;5.76,-38.06,;16.41,-36.49,;17.75,-35.72,;17.75,-34.18,;19.08,-36.49,;19.07,-38.03,;20.4,-38.8,;21.74,-38.04,;21.74,-36.5,;20.41,-35.72,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566630
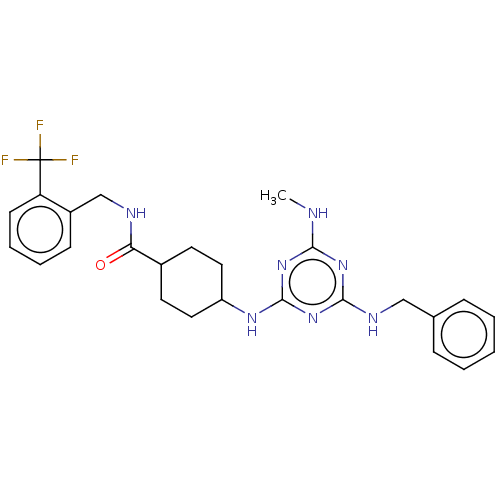
(CHEMBL4850355)Show SMILES CNc1nc(NCc2ccccc2)nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)n1 |(69.68,-31.78,;68.35,-32.56,;68.35,-34.1,;69.7,-34.87,;69.7,-36.41,;71.03,-37.18,;72.36,-36.41,;73.7,-37.18,;73.69,-38.72,;75.02,-39.49,;76.36,-38.72,;76.35,-37.17,;75.02,-36.41,;68.36,-37.18,;67.03,-36.41,;65.7,-37.18,;64.37,-36.41,;63.03,-37.17,;61.7,-36.41,;61.7,-34.87,;63.03,-34.09,;64.37,-34.86,;60.37,-34.11,;60.36,-32.57,;59.03,-34.88,;57.7,-34.12,;56.37,-34.89,;55.03,-34.13,;53.7,-34.9,;53.7,-36.44,;55.04,-37.21,;56.37,-36.44,;57.7,-37.21,;58.47,-35.87,;59.24,-37.2,;57.71,-38.75,;67.03,-34.87,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566622
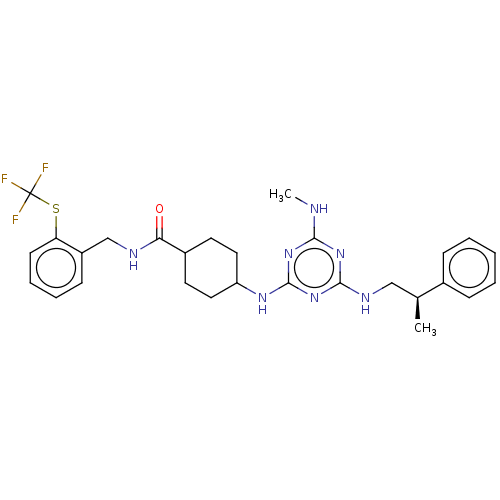
(CHEMBL4847993)Show SMILES CNc1nc(NC[C@H](C)c2ccccc2)nc(NC2CCC(CC2)C(=O)NCc2ccccc2SC(F)(F)F)n1 |r,wU:7.7,(20.04,-50.22,;18.71,-51,;18.72,-52.54,;20.06,-53.31,;20.06,-54.85,;21.39,-55.62,;22.73,-54.85,;24.06,-55.62,;24.06,-57.16,;25.39,-54.85,;26.72,-55.62,;28.06,-54.86,;28.06,-53.31,;26.72,-52.54,;25.39,-53.32,;18.73,-55.62,;17.4,-54.85,;16.06,-55.62,;14.73,-54.85,;13.39,-55.61,;12.07,-54.85,;12.06,-53.31,;13.39,-52.53,;14.73,-53.3,;10.73,-52.55,;10.72,-51.01,;9.4,-53.32,;8.06,-52.56,;6.73,-53.33,;5.39,-52.57,;4.07,-53.34,;4.06,-54.88,;5.4,-55.65,;6.73,-54.88,;8.07,-55.65,;8.07,-57.19,;7.29,-58.52,;6.52,-57.18,;9.41,-57.96,;17.39,-53.31,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566621

(CHEMBL4871040)Show SMILES CC(C)CNc1nc(NC[C@H](C)c2ccccc2)nc(NC2CCC(CC2)C(=O)NCc2ccc(cc2)C(O)=O)n1 |r,wU:10.10,(56.09,-33.98,;54.76,-34.75,;53.42,-33.99,;54.76,-36.29,;53.43,-37.07,;53.44,-38.61,;54.78,-39.38,;54.78,-40.92,;56.11,-41.69,;57.45,-40.92,;58.78,-41.69,;58.78,-43.23,;60.11,-40.92,;61.44,-41.7,;62.78,-40.93,;62.78,-39.39,;61.44,-38.62,;60.11,-39.39,;53.45,-41.69,;52.12,-40.92,;50.78,-41.69,;49.45,-40.92,;48.11,-41.68,;46.79,-40.92,;46.78,-39.38,;48.11,-38.6,;49.45,-39.37,;45.45,-38.62,;45.44,-37.08,;44.12,-39.39,;42.78,-38.63,;41.45,-39.4,;41.45,-40.95,;40.12,-41.72,;38.78,-40.95,;38.78,-39.41,;40.11,-38.64,;37.45,-41.72,;36.12,-40.95,;37.45,-43.26,;52.11,-39.38,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566624

(CHEMBL4851172)Show SMILES CNc1nc(NC[C@H](C)c2ccccc2)nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)n1 |r,wU:7.7,(18.77,-3.6,;17.44,-4.37,;17.44,-5.91,;18.78,-6.68,;18.78,-8.22,;20.12,-8.99,;21.45,-8.22,;22.78,-8.99,;22.78,-10.53,;24.12,-8.22,;25.45,-9,;26.78,-8.23,;26.78,-6.69,;25.44,-5.92,;24.11,-6.69,;17.45,-8.99,;16.12,-8.22,;14.79,-8.99,;13.45,-8.22,;12.12,-8.99,;10.79,-8.22,;10.79,-6.69,;12.12,-5.9,;13.45,-6.68,;9.45,-5.92,;9.45,-4.38,;8.12,-6.7,;6.78,-5.93,;5.45,-6.71,;4.12,-5.94,;2.79,-6.71,;2.79,-8.26,;4.12,-9.03,;5.46,-8.26,;6.79,-9.02,;7.55,-7.68,;8.33,-9.01,;6.8,-10.56,;16.11,-6.68,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566619

(CHEMBL4871498)Show SMILES C[C@@H](CNc1nc(NCCc2cccs2)nc(NC2CCC(CC2)C(=O)NCc2ccc(cc2)C(O)=O)n1)c1ccccc1 |r,wU:1.0,(24.78,-28.42,;24.78,-26.88,;23.45,-26.11,;22.12,-26.88,;20.78,-26.11,;20.78,-24.57,;19.44,-23.8,;19.43,-22.26,;20.77,-21.48,;20.76,-19.94,;22.09,-19.17,;22.25,-17.63,;23.75,-17.31,;24.53,-18.64,;23.5,-19.78,;18.11,-24.57,;18.12,-26.11,;16.79,-26.88,;15.45,-26.11,;14.12,-26.88,;12.79,-26.11,;12.79,-24.57,;14.11,-23.79,;15.45,-24.57,;11.45,-23.81,;11.45,-22.27,;10.12,-24.58,;8.78,-23.82,;7.45,-24.59,;7.46,-26.14,;6.12,-26.92,;4.79,-26.15,;4.79,-24.6,;6.12,-23.83,;3.45,-26.91,;2.12,-26.14,;3.45,-28.45,;19.45,-26.88,;26.12,-26.11,;27.45,-26.89,;28.78,-26.12,;28.78,-24.58,;27.44,-23.81,;26.11,-24.58,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566633

(CHEMBL4873163)Show SMILES CNc1nc(Nc2ccc(cc2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566625

(CHEMBL4875310)Show SMILES CNc1nc(NC[C@H](C)c2ccccc2)nc(NC2CCC(CC2)C(=O)NCc2ccc(cc2)C(F)(F)F)n1 |r,wU:7.7,(60.82,-3,;59.49,-3.77,;59.5,-5.31,;60.84,-6.08,;60.84,-7.62,;62.17,-8.39,;63.51,-7.62,;64.84,-8.39,;64.84,-9.93,;66.17,-7.63,;67.51,-8.4,;68.84,-7.63,;68.84,-6.09,;67.5,-5.32,;66.17,-6.09,;59.51,-8.39,;58.18,-7.62,;56.84,-8.39,;55.51,-7.62,;54.17,-8.39,;52.84,-7.62,;52.84,-6.09,;54.17,-5.3,;55.51,-6.08,;51.5,-5.32,;51.5,-3.78,;50.18,-6.1,;48.84,-5.33,;47.51,-6.11,;47.52,-7.66,;46.18,-8.43,;44.84,-7.66,;44.84,-6.11,;46.17,-5.34,;43.5,-8.42,;44.27,-9.75,;42.73,-9.75,;42.17,-7.66,;58.17,-6.08,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566626

(CHEMBL4853643)Show SMILES CNc1nc(NC[C@H](C)c2ccccc2)nc(NC2CCC(CC2)C(=O)NCc2ccccc2)n1 |r,wU:7.7,(19.18,-17.88,;17.85,-18.65,;17.85,-20.19,;19.2,-20.96,;19.2,-22.5,;20.53,-23.27,;21.86,-22.5,;23.2,-23.27,;23.2,-24.81,;24.53,-22.5,;25.86,-23.28,;27.19,-22.51,;27.2,-20.97,;25.85,-20.2,;24.53,-20.97,;17.86,-23.27,;16.53,-22.5,;15.2,-23.27,;13.87,-22.5,;12.53,-23.27,;11.2,-22.5,;11.2,-20.97,;12.53,-20.18,;13.86,-20.96,;9.86,-20.2,;9.86,-18.66,;8.53,-20.98,;7.2,-20.21,;5.87,-20.99,;4.53,-20.22,;3.2,-20.99,;3.2,-22.54,;4.53,-23.31,;5.87,-22.54,;16.53,-20.96,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566634

(CHEMBL4860138)Show SMILES CNc1nc(nc(n1)N1CCN(C)CC1)N1CC(C1)C(=O)NCc1ccccc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566639

(CHEMBL4869020)Show SMILES C[C@@H](CNc1ncnc(NC2CCC(CC2)C(=O)NCc2ccc(cc2)C(O)=O)n1)c1ccccc1 |r,wU:1.0,(24.78,-28.42,;24.78,-26.88,;23.45,-26.11,;22.12,-26.88,;20.78,-26.11,;20.78,-24.57,;19.44,-23.8,;18.11,-24.57,;18.12,-26.11,;16.79,-26.88,;15.45,-26.11,;14.12,-26.88,;12.79,-26.11,;12.79,-24.57,;14.11,-23.79,;15.45,-24.57,;11.45,-23.81,;11.45,-22.27,;10.12,-24.58,;8.78,-23.82,;7.45,-24.59,;7.46,-26.14,;6.12,-26.92,;4.79,-26.15,;4.79,-24.6,;6.12,-23.83,;3.45,-26.91,;2.12,-26.14,;3.45,-28.45,;19.45,-26.88,;26.12,-26.11,;27.45,-26.89,;28.78,-26.12,;28.78,-24.58,;27.44,-23.81,;26.11,-24.58,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50566633

(CHEMBL4873163)Show SMILES CNc1nc(Nc2ccc(cc2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50566633

(CHEMBL4873163)Show SMILES CNc1nc(Nc2ccc(cc2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566627

(CHEMBL4846216)Show SMILES CNc1nc(NC[C@H](C)c2ccccc2)nc(NC2CCC(CC2)C(=O)Nc2ccc(CC(O)=O)cc2)n1 |r,wU:7.7,(54.98,-18.88,;53.65,-19.65,;53.66,-21.19,;55,-21.97,;55,-23.51,;56.33,-24.28,;57.66,-23.51,;59,-24.28,;59,-25.82,;60.33,-23.51,;61.67,-24.28,;63,-23.52,;63,-21.98,;61.66,-21.2,;60.33,-21.98,;53.66,-24.28,;52.33,-23.51,;51,-24.28,;49.66,-23.51,;48.33,-24.27,;47,-23.51,;47,-21.97,;48.32,-21.19,;49.66,-21.97,;45.66,-21.21,;45.66,-19.66,;44.33,-21.98,;42.99,-21.22,;42.99,-19.68,;41.66,-18.91,;40.32,-19.69,;38.98,-18.93,;37.65,-19.7,;36.32,-18.94,;37.66,-21.24,;40.33,-21.24,;41.67,-22,;52.33,-21.97,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50566633

(CHEMBL4873163)Show SMILES CNc1nc(Nc2ccc(cc2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50566632

(CHEMBL4850549)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 |(45.76,-44.82,;44.43,-45.6,;44.44,-47.14,;43.11,-47.91,;43.12,-49.45,;41.78,-50.22,;40.45,-49.45,;39.11,-50.22,;37.79,-49.45,;37.78,-47.91,;39.11,-47.13,;40.45,-47.91,;36.45,-47.15,;36.44,-45.61,;35.12,-47.92,;33.78,-47.16,;32.45,-47.93,;31.11,-47.17,;29.79,-47.94,;29.78,-49.49,;31.12,-50.26,;32.46,-49.48,;33.79,-50.25,;34.55,-48.91,;35.33,-50.24,;33.79,-51.79,;44.45,-50.22,;45.78,-49.45,;45.78,-47.91,;47.11,-50.22,;47.1,-51.76,;48.43,-52.53,;49.77,-51.77,;51.1,-52.55,;49.77,-50.23,;48.44,-49.45,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50566631

(CHEMBL4852295)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N(C)CCN(C)C |(19.35,-45.17,;18.02,-45.94,;18.02,-47.48,;16.7,-48.25,;16.7,-49.79,;15.37,-50.56,;14.04,-49.79,;12.7,-50.56,;11.37,-49.79,;11.37,-48.26,;12.7,-47.47,;14.03,-48.25,;10.03,-47.49,;10.03,-45.95,;8.7,-48.27,;7.37,-47.5,;6.04,-48.28,;4.7,-47.51,;3.37,-48.28,;3.37,-49.83,;4.7,-50.6,;6.04,-49.83,;7.37,-50.59,;8.14,-49.26,;8.91,-50.58,;7.38,-52.13,;18.03,-50.56,;19.37,-49.79,;19.37,-48.25,;20.7,-50.56,;20.7,-52.1,;22.03,-49.79,;23.37,-50.56,;24.7,-49.79,;26.03,-50.57,;24.7,-48.25,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50566633

(CHEMBL4873163)Show SMILES CNc1nc(Nc2ccc(cc2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50566632

(CHEMBL4850549)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 |(45.76,-44.82,;44.43,-45.6,;44.44,-47.14,;43.11,-47.91,;43.12,-49.45,;41.78,-50.22,;40.45,-49.45,;39.11,-50.22,;37.79,-49.45,;37.78,-47.91,;39.11,-47.13,;40.45,-47.91,;36.45,-47.15,;36.44,-45.61,;35.12,-47.92,;33.78,-47.16,;32.45,-47.93,;31.11,-47.17,;29.79,-47.94,;29.78,-49.49,;31.12,-50.26,;32.46,-49.48,;33.79,-50.25,;34.55,-48.91,;35.33,-50.24,;33.79,-51.79,;44.45,-50.22,;45.78,-49.45,;45.78,-47.91,;47.11,-50.22,;47.1,-51.76,;48.43,-52.53,;49.77,-51.77,;51.1,-52.55,;49.77,-50.23,;48.44,-49.45,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50566631

(CHEMBL4852295)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N(C)CCN(C)C |(19.35,-45.17,;18.02,-45.94,;18.02,-47.48,;16.7,-48.25,;16.7,-49.79,;15.37,-50.56,;14.04,-49.79,;12.7,-50.56,;11.37,-49.79,;11.37,-48.26,;12.7,-47.47,;14.03,-48.25,;10.03,-47.49,;10.03,-45.95,;8.7,-48.27,;7.37,-47.5,;6.04,-48.28,;4.7,-47.51,;3.37,-48.28,;3.37,-49.83,;4.7,-50.6,;6.04,-49.83,;7.37,-50.59,;8.14,-49.26,;8.91,-50.58,;7.38,-52.13,;18.03,-50.56,;19.37,-49.79,;19.37,-48.25,;20.7,-50.56,;20.7,-52.1,;22.03,-49.79,;23.37,-50.56,;24.7,-49.79,;26.03,-50.57,;24.7,-48.25,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50566632

(CHEMBL4850549)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 |(45.76,-44.82,;44.43,-45.6,;44.44,-47.14,;43.11,-47.91,;43.12,-49.45,;41.78,-50.22,;40.45,-49.45,;39.11,-50.22,;37.79,-49.45,;37.78,-47.91,;39.11,-47.13,;40.45,-47.91,;36.45,-47.15,;36.44,-45.61,;35.12,-47.92,;33.78,-47.16,;32.45,-47.93,;31.11,-47.17,;29.79,-47.94,;29.78,-49.49,;31.12,-50.26,;32.46,-49.48,;33.79,-50.25,;34.55,-48.91,;35.33,-50.24,;33.79,-51.79,;44.45,-50.22,;45.78,-49.45,;45.78,-47.91,;47.11,-50.22,;47.1,-51.76,;48.43,-52.53,;49.77,-51.77,;51.1,-52.55,;49.77,-50.23,;48.44,-49.45,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50566633

(CHEMBL4873163)Show SMILES CNc1nc(Nc2ccc(cc2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50435763

(CHEMBL2392711)Show SMILES CNc1nc(nc(n1)N1CCN(C)CC1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C23H31F3N8O/c1-27-20-29-21(31-22(30-20)34-13-11-32(2)12-14-34)33-9-7-16(8-10-33)19(35)28-15-17-5-3-4-6-18(17)23(24,25)26/h3-6,16H,7-15H2,1-2H3,(H,28,35)(H,27,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50435763

(CHEMBL2392711)Show SMILES CNc1nc(nc(n1)N1CCN(C)CC1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C23H31F3N8O/c1-27-20-29-21(31-22(30-20)34-13-11-32(2)12-14-34)33-9-7-16(8-10-33)19(35)28-15-17-5-3-4-6-18(17)23(24,25)26/h3-6,16H,7-15H2,1-2H3,(H,28,35)(H,27,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50566633

(CHEMBL4873163)Show SMILES CNc1nc(Nc2ccc(cc2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50566632

(CHEMBL4850549)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 |(45.76,-44.82,;44.43,-45.6,;44.44,-47.14,;43.11,-47.91,;43.12,-49.45,;41.78,-50.22,;40.45,-49.45,;39.11,-50.22,;37.79,-49.45,;37.78,-47.91,;39.11,-47.13,;40.45,-47.91,;36.45,-47.15,;36.44,-45.61,;35.12,-47.92,;33.78,-47.16,;32.45,-47.93,;31.11,-47.17,;29.79,-47.94,;29.78,-49.49,;31.12,-50.26,;32.46,-49.48,;33.79,-50.25,;34.55,-48.91,;35.33,-50.24,;33.79,-51.79,;44.45,-50.22,;45.78,-49.45,;45.78,-47.91,;47.11,-50.22,;47.1,-51.76,;48.43,-52.53,;49.77,-51.77,;51.1,-52.55,;49.77,-50.23,;48.44,-49.45,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50566631

(CHEMBL4852295)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N(C)CCN(C)C |(19.35,-45.17,;18.02,-45.94,;18.02,-47.48,;16.7,-48.25,;16.7,-49.79,;15.37,-50.56,;14.04,-49.79,;12.7,-50.56,;11.37,-49.79,;11.37,-48.26,;12.7,-47.47,;14.03,-48.25,;10.03,-47.49,;10.03,-45.95,;8.7,-48.27,;7.37,-47.5,;6.04,-48.28,;4.7,-47.51,;3.37,-48.28,;3.37,-49.83,;4.7,-50.6,;6.04,-49.83,;7.37,-50.59,;8.14,-49.26,;8.91,-50.58,;7.38,-52.13,;18.03,-50.56,;19.37,-49.79,;19.37,-48.25,;20.7,-50.56,;20.7,-52.1,;22.03,-49.79,;23.37,-50.56,;24.7,-49.79,;26.03,-50.57,;24.7,-48.25,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50435763

(CHEMBL2392711)Show SMILES CNc1nc(nc(n1)N1CCN(C)CC1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C23H31F3N8O/c1-27-20-29-21(31-22(30-20)34-13-11-32(2)12-14-34)33-9-7-16(8-10-33)19(35)28-15-17-5-3-4-6-18(17)23(24,25)26/h3-6,16H,7-15H2,1-2H3,(H,28,35)(H,27,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50566632

(CHEMBL4850549)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 |(45.76,-44.82,;44.43,-45.6,;44.44,-47.14,;43.11,-47.91,;43.12,-49.45,;41.78,-50.22,;40.45,-49.45,;39.11,-50.22,;37.79,-49.45,;37.78,-47.91,;39.11,-47.13,;40.45,-47.91,;36.45,-47.15,;36.44,-45.61,;35.12,-47.92,;33.78,-47.16,;32.45,-47.93,;31.11,-47.17,;29.79,-47.94,;29.78,-49.49,;31.12,-50.26,;32.46,-49.48,;33.79,-50.25,;34.55,-48.91,;35.33,-50.24,;33.79,-51.79,;44.45,-50.22,;45.78,-49.45,;45.78,-47.91,;47.11,-50.22,;47.1,-51.76,;48.43,-52.53,;49.77,-51.77,;51.1,-52.55,;49.77,-50.23,;48.44,-49.45,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50566631

(CHEMBL4852295)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N(C)CCN(C)C |(19.35,-45.17,;18.02,-45.94,;18.02,-47.48,;16.7,-48.25,;16.7,-49.79,;15.37,-50.56,;14.04,-49.79,;12.7,-50.56,;11.37,-49.79,;11.37,-48.26,;12.7,-47.47,;14.03,-48.25,;10.03,-47.49,;10.03,-45.95,;8.7,-48.27,;7.37,-47.5,;6.04,-48.28,;4.7,-47.51,;3.37,-48.28,;3.37,-49.83,;4.7,-50.6,;6.04,-49.83,;7.37,-50.59,;8.14,-49.26,;8.91,-50.58,;7.38,-52.13,;18.03,-50.56,;19.37,-49.79,;19.37,-48.25,;20.7,-50.56,;20.7,-52.1,;22.03,-49.79,;23.37,-50.56,;24.7,-49.79,;26.03,-50.57,;24.7,-48.25,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using 7-benzyloxyquinoline as substrate |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50435763

(CHEMBL2392711)Show SMILES CNc1nc(nc(n1)N1CCN(C)CC1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C23H31F3N8O/c1-27-20-29-21(31-22(30-20)34-13-11-32(2)12-14-34)33-9-7-16(8-10-33)19(35)28-15-17-5-3-4-6-18(17)23(24,25)26/h3-6,16H,7-15H2,1-2H3,(H,28,35)(H,27,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) using diethoxyfluorescein as substrate |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50435763

(CHEMBL2392711)Show SMILES CNc1nc(nc(n1)N1CCN(C)CC1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C23H31F3N8O/c1-27-20-29-21(31-22(30-20)34-13-11-32(2)12-14-34)33-9-7-16(8-10-33)19(35)28-15-17-5-3-4-6-18(17)23(24,25)26/h3-6,16H,7-15H2,1-2H3,(H,28,35)(H,27,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50566632

(CHEMBL4850549)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 |(45.76,-44.82,;44.43,-45.6,;44.44,-47.14,;43.11,-47.91,;43.12,-49.45,;41.78,-50.22,;40.45,-49.45,;39.11,-50.22,;37.79,-49.45,;37.78,-47.91,;39.11,-47.13,;40.45,-47.91,;36.45,-47.15,;36.44,-45.61,;35.12,-47.92,;33.78,-47.16,;32.45,-47.93,;31.11,-47.17,;29.79,-47.94,;29.78,-49.49,;31.12,-50.26,;32.46,-49.48,;33.79,-50.25,;34.55,-48.91,;35.33,-50.24,;33.79,-51.79,;44.45,-50.22,;45.78,-49.45,;45.78,-47.91,;47.11,-50.22,;47.1,-51.76,;48.43,-52.53,;49.77,-51.77,;51.1,-52.55,;49.77,-50.23,;48.44,-49.45,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50566631

(CHEMBL4852295)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N(C)CCN(C)C |(19.35,-45.17,;18.02,-45.94,;18.02,-47.48,;16.7,-48.25,;16.7,-49.79,;15.37,-50.56,;14.04,-49.79,;12.7,-50.56,;11.37,-49.79,;11.37,-48.26,;12.7,-47.47,;14.03,-48.25,;10.03,-47.49,;10.03,-45.95,;8.7,-48.27,;7.37,-47.5,;6.04,-48.28,;4.7,-47.51,;3.37,-48.28,;3.37,-49.83,;4.7,-50.6,;6.04,-49.83,;7.37,-50.59,;8.14,-49.26,;8.91,-50.58,;7.38,-52.13,;18.03,-50.56,;19.37,-49.79,;19.37,-48.25,;20.7,-50.56,;20.7,-52.1,;22.03,-49.79,;23.37,-50.56,;24.7,-49.79,;26.03,-50.57,;24.7,-48.25,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50566631

(CHEMBL4852295)Show SMILES CNc1nc(NC2CCC(CC2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N(C)CCN(C)C |(19.35,-45.17,;18.02,-45.94,;18.02,-47.48,;16.7,-48.25,;16.7,-49.79,;15.37,-50.56,;14.04,-49.79,;12.7,-50.56,;11.37,-49.79,;11.37,-48.26,;12.7,-47.47,;14.03,-48.25,;10.03,-47.49,;10.03,-45.95,;8.7,-48.27,;7.37,-47.5,;6.04,-48.28,;4.7,-47.51,;3.37,-48.28,;3.37,-49.83,;4.7,-50.6,;6.04,-49.83,;7.37,-50.59,;8.14,-49.26,;8.91,-50.58,;7.38,-52.13,;18.03,-50.56,;19.37,-49.79,;19.37,-48.25,;20.7,-50.56,;20.7,-52.1,;22.03,-49.79,;23.37,-50.56,;24.7,-49.79,;26.03,-50.57,;24.7,-48.25,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50435763

(CHEMBL2392711)Show SMILES CNc1nc(nc(n1)N1CCN(C)CC1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C23H31F3N8O/c1-27-20-29-21(31-22(30-20)34-13-11-32(2)12-14-34)33-9-7-16(8-10-33)19(35)28-15-17-5-3-4-6-18(17)23(24,25)26/h3-6,16H,7-15H2,1-2H3,(H,28,35)(H,27,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data