

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus B) | BDBM50137747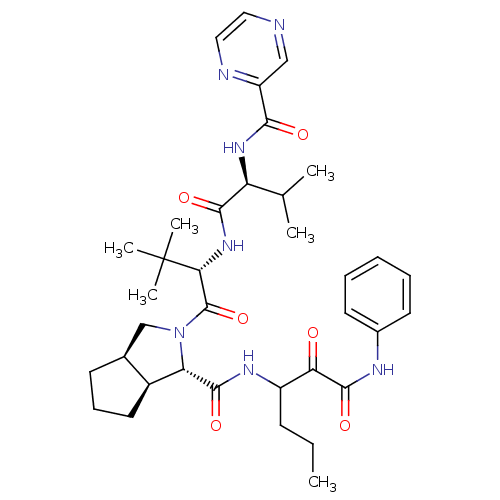 ((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137739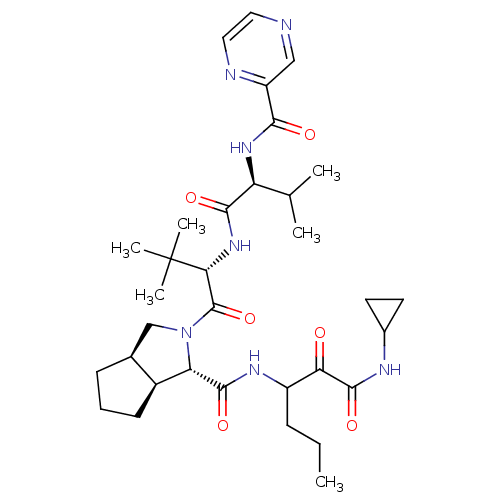 ((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137748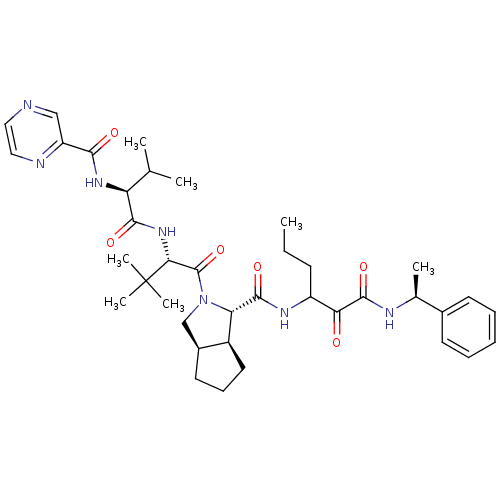 ((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(3S,4S)-3-meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137744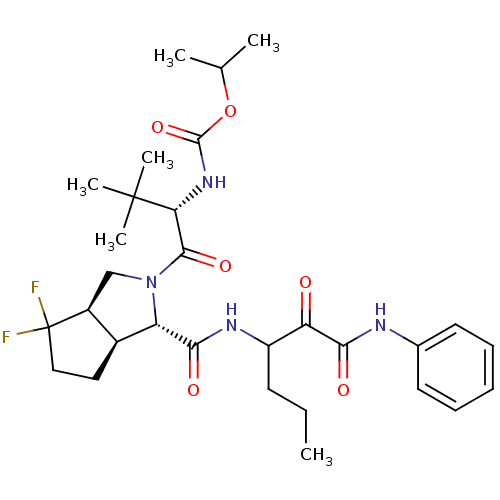 (CHEMBL328740 | {(S)-1-[(1S,3aR,6aS)-4,4-Difluoro-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137741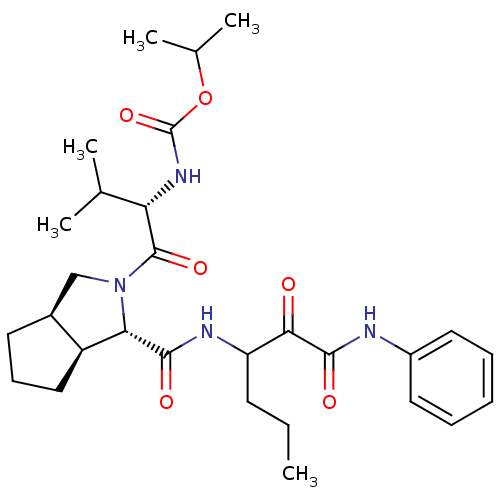 (CHEMBL84855 | {(S)-2-Methyl-1-[(1S,3aR,6aS)-1-(1-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137740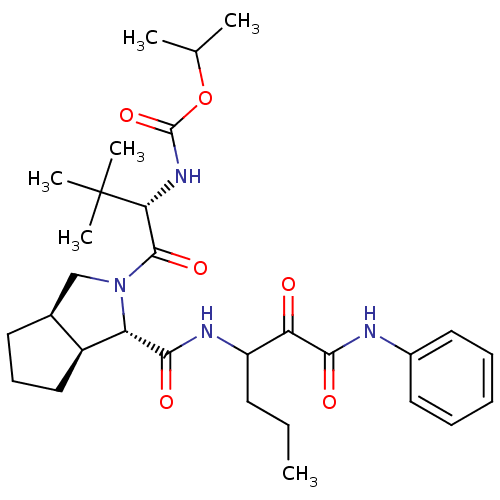 (CHEMBL89106 | {(S)-2,2-Dimethyl-1-[(1S,3aR,6aS)-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133641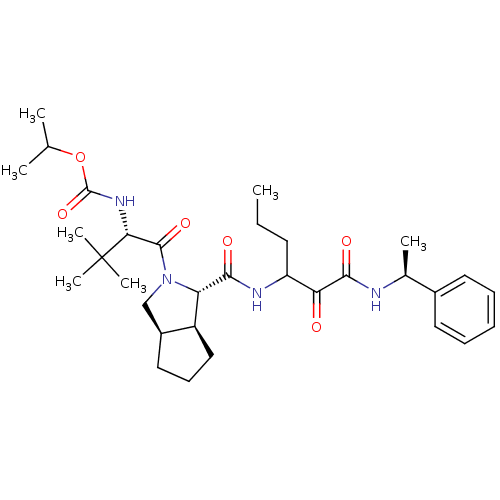 (((S)-2,2-Dimethyl-1-{(1S,3aR,6aS)-1-[1-((S)-1-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137742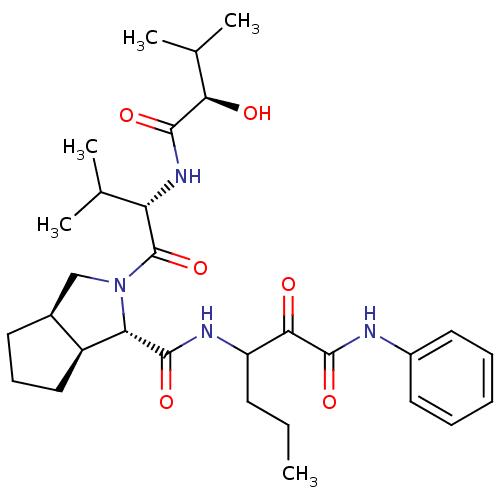 ((1S,3aR,6aS)-2-[(S)-2-((R)-2-Hydroxy-3-methyl-buty...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133645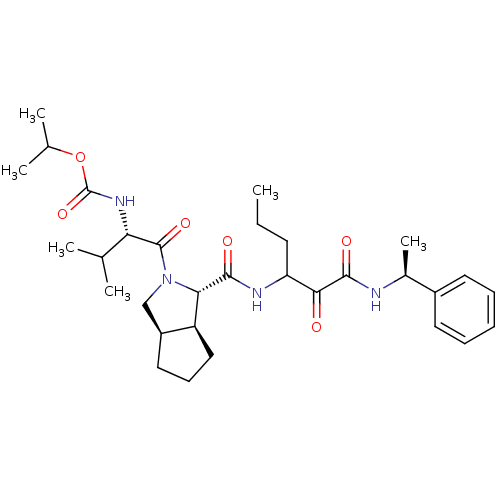 (((S)-2-Methyl-1-{(1S,3aR,6aS)-1-[1-((S)-1-phenyl-e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137743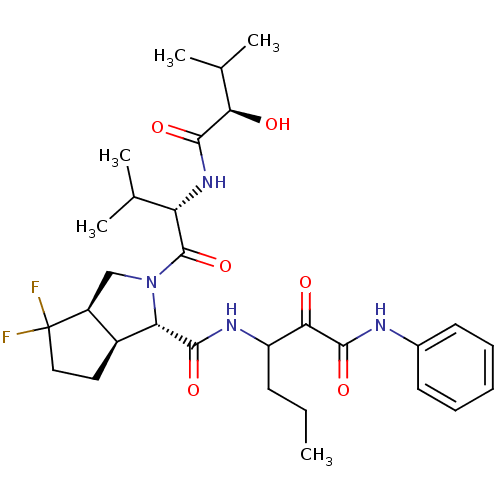 ((1S,3aR,6aS)-4,4-Difluoro-2-[(S)-2-((R)-2-hydroxy-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137750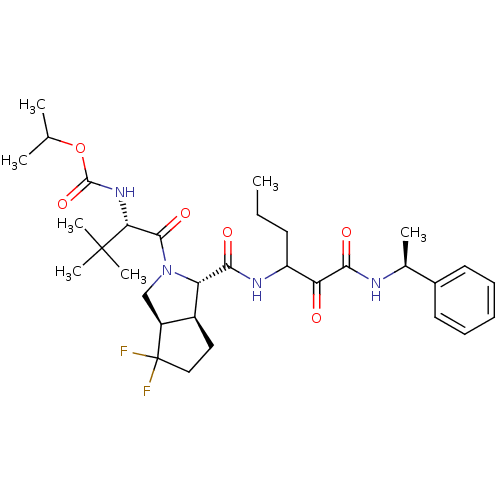 (((S)-1-{(1S,3aR,6aS)-4,4-Difluoro-1-[1-((S)-1-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137749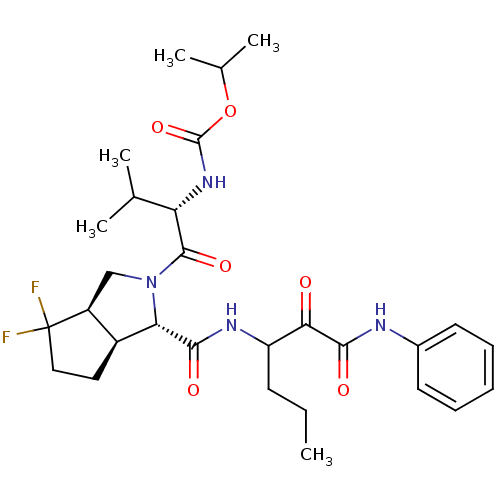 (CHEMBL88470 | {(S)-1-[(1S,3aR,6aS)-4,4-Difluoro-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137746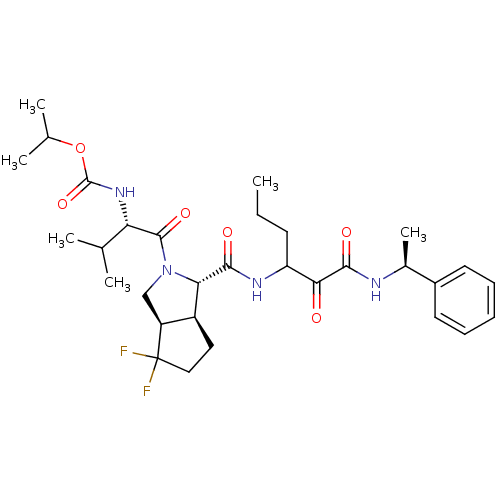 (((S)-1-{(1S,3aR,6aS)-4,4-Difluoro-1-[1-((S)-1-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137745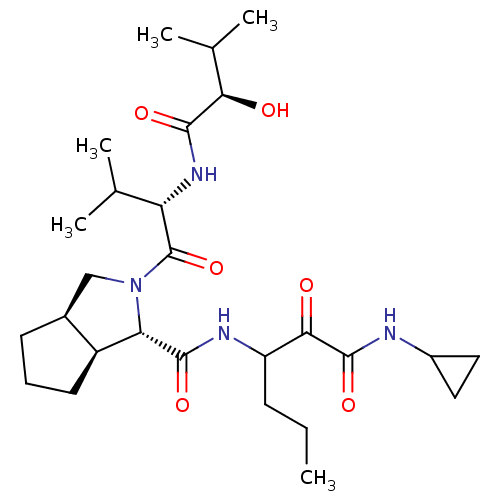 ((1S,3aR,6aS)-2-[(S)-2-((R)-2-Hydroxy-3-methyl-buty...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137751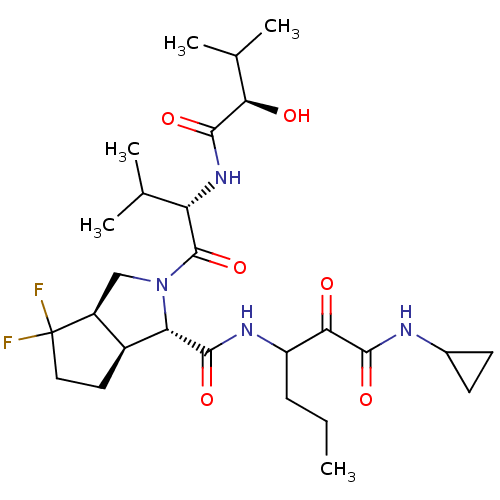 ((1S,3aR,6aS)-4,4-Difluoro-2-[(S)-2-((R)-2-hydroxy-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137748 ((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(3S,4S)-3-meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137739 ((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137744 (CHEMBL328740 | {(S)-1-[(1S,3aR,6aS)-4,4-Difluoro-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Protease using replicon assay in rats at 50 microM concentration | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137741 (CHEMBL84855 | {(S)-2-Methyl-1-[(1S,3aR,6aS)-1-(1-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137749 (CHEMBL88470 | {(S)-1-[(1S,3aR,6aS)-4,4-Difluoro-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133641 (((S)-2,2-Dimethyl-1-{(1S,3aR,6aS)-1-[1-((S)-1-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137745 ((1S,3aR,6aS)-2-[(S)-2-((R)-2-Hydroxy-3-methyl-buty...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137747 ((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137746 (((S)-1-{(1S,3aR,6aS)-4,4-Difluoro-1-[1-((S)-1-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Protease using replicon assay in rats at 50 microM concentration | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137743 ((1S,3aR,6aS)-4,4-Difluoro-2-[(S)-2-((R)-2-hydroxy-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Protease using replicon assay in rats at 50 microM concentration | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137739 ((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137748 ((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(3S,4S)-3-meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137750 (((S)-1-{(1S,3aR,6aS)-4,4-Difluoro-1-[1-((S)-1-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137742 ((1S,3aR,6aS)-2-[(S)-2-((R)-2-Hydroxy-3-methyl-buty...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50133645 (((S)-2-Methyl-1-{(1S,3aR,6aS)-1-[1-((S)-1-phenyl-e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 263-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0FX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||