 Found 16 hits Enz. Inhib. hit(s) with all data for entry = 50014163
Found 16 hits Enz. Inhib. hit(s) with all data for entry = 50014163 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138930
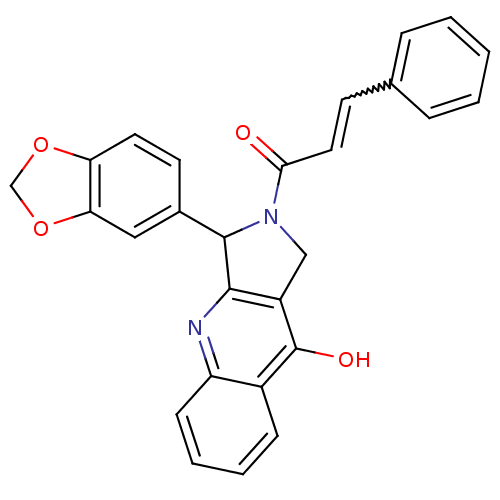
(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-acryloyl)-1,2,...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)C=Cc1ccccc1 |w:26.31| Show InChI InChI=1S/C27H20N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-14,26H,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138939
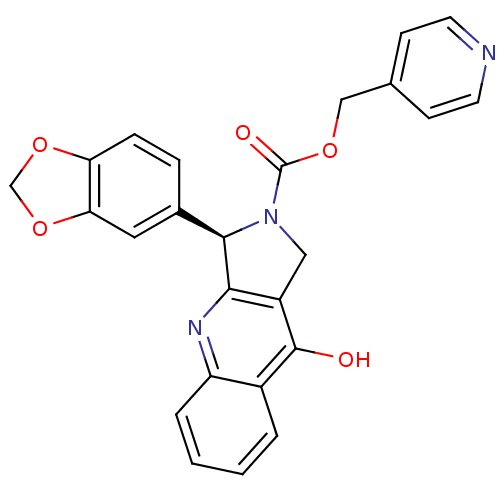
((R)-3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahyd...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138929

(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138936
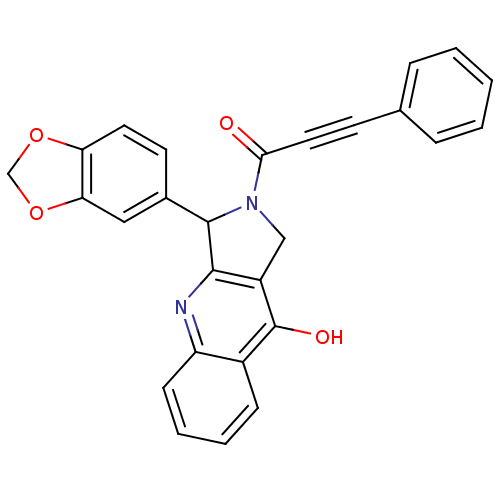
(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-propynoyl)-1,2...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)C#Cc1ccccc1 Show InChI InChI=1S/C27H18N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-9,11-12,14,26H,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138927
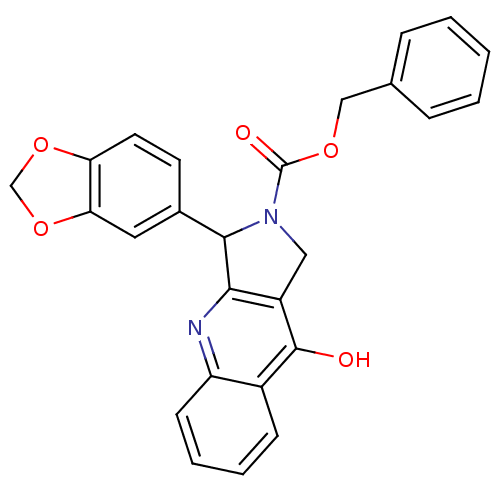
(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H20N2O5/c29-25-18-8-4-5-9-20(18)27-23-19(25)13-28(26(30)31-14-16-6-2-1-3-7-16)24(23)17-10-11-21-22(12-17)33-15-32-21/h1-12,24H,13-15H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138935

(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C26H21N3O4/c30-25-18-8-4-5-9-20(18)28-23-19(25)14-29(26(31)27-13-16-6-2-1-3-7-16)24(23)17-10-11-21-22(12-17)33-15-32-21/h1-12,24H,13-15H2,(H,27,31)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122979
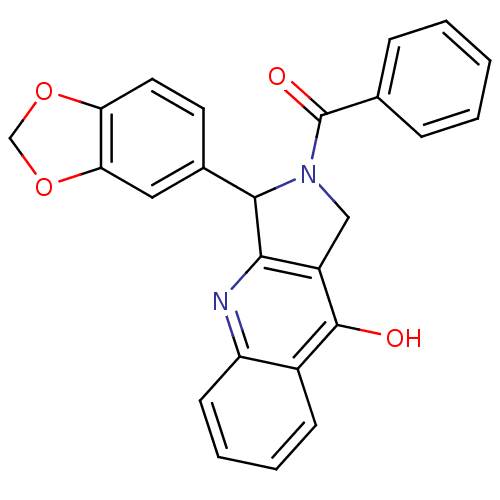
(3-Benzo[1,3]dioxol-5-yl-2-benzoyl-1,2,3,4-tetrahyd...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H18N2O4/c28-24-17-8-4-5-9-19(17)26-22-18(24)13-27(25(29)15-6-2-1-3-7-15)23(22)16-10-11-20-21(12-16)31-14-30-20/h1-12,23H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138928
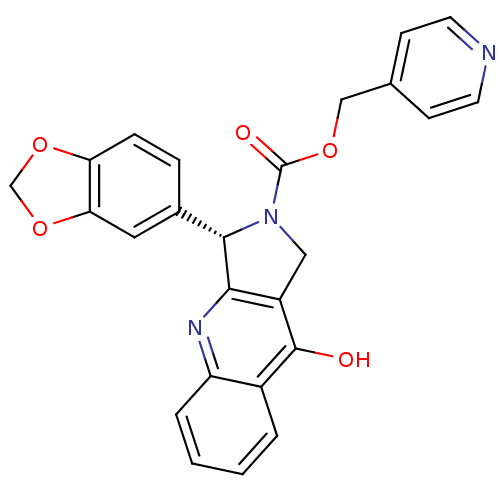
((S)-3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahyd...)Show SMILES Oc1c2CN([C@H](c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138933

(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-propionyl)-1,2...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)CCc1ccccc1 Show InChI InChI=1S/C27H22N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-9,11-12,14,26H,10,13,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138931

(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES CN(Cc1ccccc1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C27H23N3O4/c1-29(14-17-7-3-2-4-8-17)27(32)30-15-20-24(28-21-10-6-5-9-19(21)26(20)31)25(30)18-11-12-22-23(13-18)34-16-33-22/h2-13,25H,14-16H2,1H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138932

(3-Benzo[1,3]dioxol-5-yl-2-phenylacetyl-1,2,3,4-tet...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c29-23(12-16-6-2-1-3-7-16)28-14-19-24(27-20-9-5-4-8-18(20)26(19)30)25(28)17-10-11-21-22(13-17)32-15-31-21/h1-11,13,25H,12,14-15H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138940

(3-Benzo[1,3]dioxol-5-yl-2-((E)-3-phenyl-allyl)-1,2...)Show SMILES Oc1c2CN(C\C=C\c3ccccc3)C(c2nc2ccccc12)c1ccc2OCOc2c1 Show InChI InChI=1S/C27H22N2O3/c30-27-20-10-4-5-11-22(20)28-25-21(27)16-29(14-6-9-18-7-2-1-3-8-18)26(25)19-12-13-23-24(15-19)32-17-31-23/h1-13,15,26H,14,16-17H2,(H,28,30)/b9-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138937
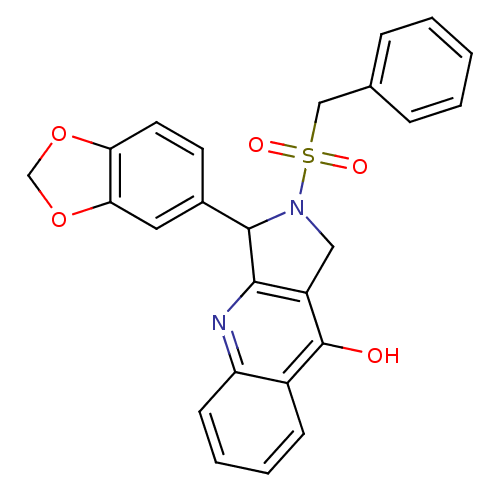
(3-Benzo[1,3]dioxol-5-yl-2-phenylmethanesulfonyl-1,...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C25H20N2O5S/c28-25-18-8-4-5-9-20(18)26-23-19(25)13-27(33(29,30)14-16-6-2-1-3-7-16)24(23)17-10-11-21-22(12-17)32-15-31-21/h1-12,24H,13-15H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138938
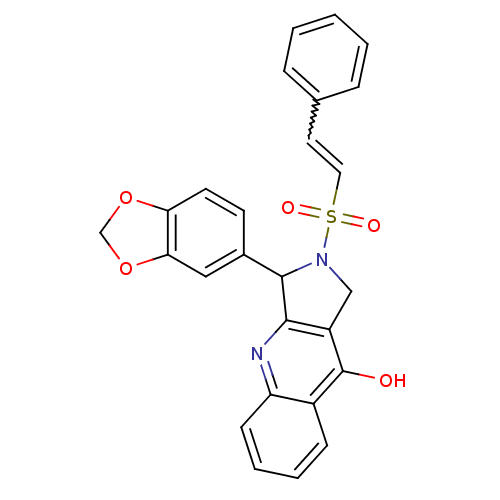
(3-Benzo[1,3]dioxol-5-yl-2-(2-phenyl-ethenesulfonyl...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)S(=O)(=O)C=Cc1ccccc1 |w:27.32| Show InChI InChI=1S/C26H20N2O5S/c29-26-19-8-4-5-9-21(19)27-24-20(26)15-28(34(30,31)13-12-17-6-2-1-3-7-17)25(24)18-10-11-22-23(14-18)33-16-32-22/h1-14,25H,15-16H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138934

(3-Benzo[1,3]dioxol-5-yl-4-methyl-9-oxo-1,3,4,9-tet...)Show SMILES Cn1c2C(N(Cc2c(=O)c2ccccc12)C(=O)OCc1ccncc1)c1ccc2OCOc2c1 Show InChI InChI=1S/C26H21N3O5/c1-28-20-5-3-2-4-18(20)25(30)19-13-29(26(31)32-14-16-8-10-27-11-9-16)23(24(19)28)17-6-7-21-22(12-17)34-15-33-21/h2-12,23H,13-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 601 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data