 Found 25 hits Enz. Inhib. hit(s) with all data for entry = 50015331
Found 25 hits Enz. Inhib. hit(s) with all data for entry = 50015331 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154646

(4N-methyl-4N-phenyl-1-{3-[3-(4-methylanilino-1-pyr...)Show SMILES C[N+](c1ccccc1)=c1ccn(Cc2cccc(c2)-c2cccc(Cn3ccc(cc3)=[N+](C)c3ccccc3)c2)cc1 |(-11.04,1.1,;-9.69,1.83,;-9.62,3.35,;-8.27,4.07,;-8.2,5.61,;-9.5,6.44,;-10.86,5.71,;-10.92,4.17,;-8.39,1.01,;-8.44,-.53,;-7.12,-1.35,;-5.76,-.64,;-4.22,-.61,;-3.44,-1.95,;-4.2,-3.28,;-3.44,-4.62,;-1.9,-4.62,;-1.13,-3.28,;-1.9,-1.95,;.41,-3.28,;1.18,-4.62,;2.71,-4.62,;3.48,-3.28,;2.71,-1.94,;3.48,-.6,;5,-.6,;5.78,-1.93,;7.32,-1.93,;8.09,-.58,;7.3,.76,;5.75,.74,;9.63,-.58,;10.41,-1.91,;10.39,.76,;11.91,.76,;12.65,2.07,;11.9,3.39,;10.36,3.37,;9.61,2.04,;1.17,-1.95,;-5.7,.89,;-7.02,1.73,)| Show InChI InChI=1S/C38H36N4/c1-39(35-15-5-3-6-16-35)37-19-23-41(24-20-37)29-31-11-9-13-33(27-31)34-14-10-12-32(28-34)30-42-25-21-38(22-26-42)40(2)36-17-7-4-8-18-36/h3-28H,29-30H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154653
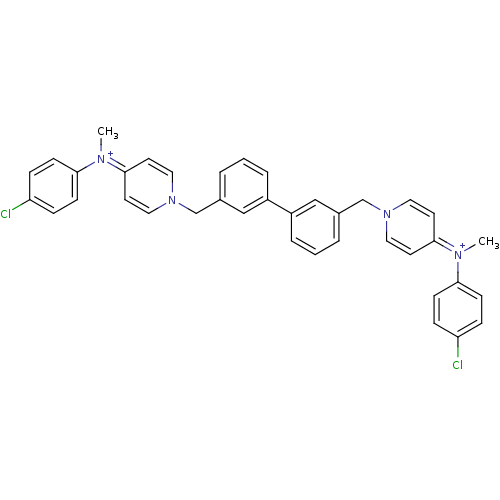
(4N-(4-chlorophenyl)-4N-methyl-1-[3-(3-{4-[4-chloro...)Show SMILES C[N+](c1ccc(Cl)cc1)=c1ccn(Cc2cccc(c2)-c2cccc(Cn3ccc(cc3)=[N+](C)c3ccc(Cl)cc3)c2)cc1 |(-11.11,.84,;-9.76,1.56,;-9.69,3.08,;-8.32,3.8,;-8.27,5.35,;-9.57,6.15,;-9.52,7.69,;-10.93,5.43,;-10.99,3.9,;-8.44,.72,;-7.09,1.44,;-5.77,.62,;-5.83,-.92,;-4.29,-.9,;-3.51,-2.23,;-4.27,-3.55,;-3.51,-4.9,;-1.97,-4.9,;-1.2,-3.55,;-1.97,-2.23,;.35,-3.55,;1.11,-4.9,;2.64,-4.9,;3.43,-3.55,;2.64,-2.23,;3.39,-.88,;4.93,-.88,;5.68,.46,;7.22,.47,;8.02,-.85,;7.25,-2.2,;5.7,-2.2,;9.56,-.85,;10.33,-2.18,;10.3,.47,;11.83,.47,;12.58,1.8,;11.82,3.13,;12.58,4.46,;10.29,3.09,;9.54,1.76,;1.09,-2.23,;-7.19,-1.62,;-8.51,-.8,)| Show InChI InChI=1S/C38H34Cl2N4/c1-41(35-13-9-33(39)10-14-35)37-17-21-43(22-18-37)27-29-5-3-7-31(25-29)32-8-4-6-30(26-32)28-44-23-19-38(20-24-44)42(2)36-15-11-34(40)12-16-36/h3-26H,27-28H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154664

(4N-(4-chlorophenyl)-4N-methyl-1-[4-(4-{4-[4-chloro...)Show SMILES C[N+](c1ccc(Cl)cc1)=c1ccn(Cc2ccc(cc2)-c2ccc(Cn3ccc(cc3)=[N+](C)c3ccc(Cl)cc3)cc2)cc1 |(-8.94,2.54,;-9,1.01,;-10.35,.25,;-10.4,-1.28,;-11.76,-2.01,;-13.07,-1.2,;-14.43,-1.93,;-13.02,.36,;-11.68,1.07,;-7.7,.17,;-6.34,.91,;-5.01,.1,;-5.07,-1.44,;-4.27,-2.77,;-2.72,-2.77,;-1.97,-4.09,;-.43,-4.09,;.35,-2.77,;-.43,-1.44,;-1.97,-1.44,;1.88,-2.77,;2.66,-4.09,;4.2,-4.09,;4.98,-2.77,;6.52,-2.77,;7.29,-1.44,;6.52,-.1,;7.27,1.22,;8.82,1.22,;9.59,-.1,;8.82,-1.44,;9.58,2.57,;11.12,2.57,;8.81,3.9,;9.58,5.25,;8.81,6.6,;7.27,6.6,;6.49,7.88,;6.49,5.22,;7.29,3.9,;4.2,-1.43,;2.65,-1.44,;-6.42,-2.16,;-7.72,-1.34,)| Show InChI InChI=1S/C38H34Cl2N4/c1-41(35-15-11-33(39)12-16-35)37-19-23-43(24-20-37)27-29-3-7-31(8-4-29)32-9-5-30(6-10-32)28-44-25-21-38(22-26-44)42(2)36-17-13-34(40)14-18-36/h3-26H,27-28H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154654
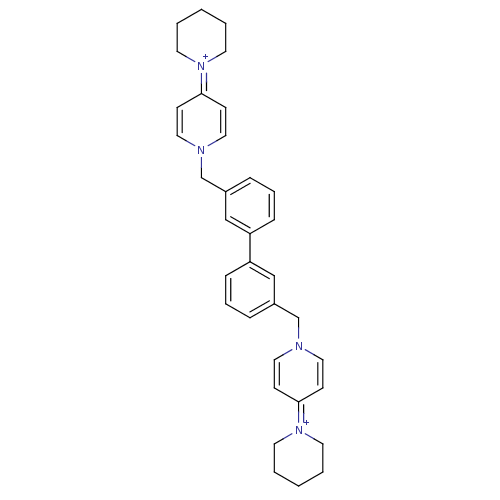
(4-hexahydro-1-pyridinyl-1-{3-[3-(4-hexahydro-1-pyr...)Show SMILES C(c1cccc(c1)-c1cccc(Cn2ccc(cc2)=[N+]2CCCCC2)c1)n1ccc(cc1)=[N+]1CCCCC1 |(-4.03,.12,;-3.24,-1.22,;-4.01,-2.54,;-3.24,-3.86,;-1.7,-3.86,;-.93,-2.52,;-1.7,-1.2,;.61,-2.52,;1.36,-3.86,;2.88,-3.86,;3.66,-2.52,;2.88,-1.19,;3.66,.14,;5.19,.14,;5.96,-1.19,;7.5,-1.18,;8.24,.17,;7.48,1.49,;5.93,1.47,;9.78,.17,;10.56,-1.17,;12.08,-1.15,;12.84,.18,;12.07,1.49,;10.53,1.49,;1.36,-1.2,;-5.56,.12,;-6.91,-.61,;-8.23,.23,;-8.18,1.75,;-6.8,2.47,;-5.49,1.63,;-9.47,2.56,;-9.4,4.11,;-10.69,4.92,;-12.06,4.21,;-12.11,2.69,;-10.8,1.83,)| Show InChI InChI=1S/C34H40N4/c1-3-17-37(18-4-1)33-13-21-35(22-14-33)27-29-9-7-11-31(25-29)32-12-8-10-30(26-32)28-36-23-15-34(16-24-36)38-19-5-2-6-20-38/h7-16,21-26H,1-6,17-20,27-28H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154651

(4-(3,5-dichlorophenyl)-1-(3-{3-[4-(3,5-dichlorophe...)Show SMILES Clc1cc(Cl)cc(c1)-c1cc[n+](Cc2cccc(c2)-c2cccc(C[n+]3ccc(cc3)-c3cc(Cl)cc(Cl)c3)c2)cc1 Show InChI InChI=1S/C36H26Cl4N2/c37-33-17-31(18-34(38)21-33)27-7-11-41(12-8-27)23-25-3-1-5-29(15-25)30-6-2-4-26(16-30)24-42-13-9-28(10-14-42)32-19-35(39)22-36(40)20-32/h1-22H,23-24H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154644

(4N-(4-chlorophenyl)-4N-methyl-1-(4-{4-[4-chloro(me...)Show SMILES C[N+](c1ccc(Cl)cc1)=c1ccn(Cc2ccc(Cn3ccc(cc3)=[N+](C)c3ccc(Cl)cc3)cc2)cc1 |(-7.59,-.82,;-6.24,-.09,;-6.2,1.47,;-4.83,2.19,;-4.82,3.74,;-6.14,4.53,;-6.1,6.07,;-7.48,3.78,;-7.52,2.24,;-4.9,-.89,;-3.57,-.14,;-2.24,-.94,;-2.27,-2.46,;-1.5,-3.81,;.05,-3.81,;.82,-2.46,;2.36,-2.46,;3.14,-3.8,;4.68,-3.8,;5.43,-2.46,;6.77,-1.71,;6.77,-.16,;5.42,.6,;4.09,-.17,;4.09,-1.71,;5.42,2.14,;6.77,2.92,;4.09,2.89,;2.81,2.14,;1.47,2.89,;1.46,4.42,;.12,5.18,;2.78,5.18,;4.1,4.42,;2.36,-5.12,;.82,-5.12,;-3.62,-3.21,;-4.95,-2.42,)| Show InChI InChI=1S/C32H30Cl2N4/c1-35(29-11-7-27(33)8-12-29)31-15-19-37(20-16-31)23-25-3-5-26(6-4-25)24-38-21-17-32(18-22-38)36(2)30-13-9-28(34)10-14-30/h3-22H,23-24H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154658
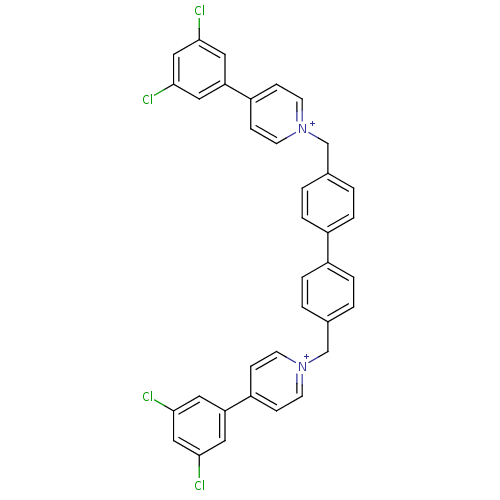
(4-(3,5-dichlorophenyl)-1-(4-{4-[4-(3,5-dichlorophe...)Show SMILES Clc1cc(Cl)cc(c1)-c1cc[n+](Cc2ccc(cc2)-c2ccc(C[n+]3ccc(cc3)-c3cc(Cl)cc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C36H26Cl4N2/c37-33-17-31(18-34(38)21-33)29-9-13-41(14-10-29)23-25-1-5-27(6-2-25)28-7-3-26(4-8-28)24-42-15-11-30(12-16-42)32-19-35(39)22-36(40)20-32/h1-22H,23-24H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154656
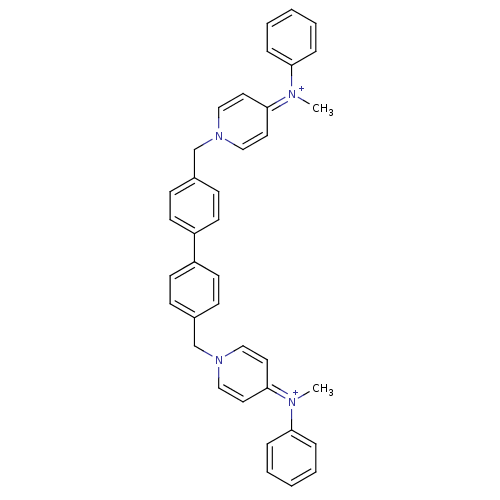
(4N-methyl-4N-phenyl-1-{4-[4-(4-methylanilino-1-pyr...)Show SMILES C[N+](c1ccccc1)=c1ccn(Cc2ccc(cc2)-c2ccc(Cn3ccc(cc3)=[N+](C)c3ccccc3)cc2)cc1 |(-9.12,2.6,;-9.17,1.07,;-10.52,.32,;-11.81,1.15,;-13.17,.43,;-13.21,-1.12,;-11.89,-1.93,;-10.57,-1.19,;-7.86,.25,;-7.91,-1.26,;-6.59,-2.07,;-5.22,-1.36,;-4.45,-2.69,;-2.92,-2.69,;-2.13,-4.02,;-.59,-4.02,;.17,-2.69,;-.59,-1.36,;-2.13,-1.36,;1.71,-2.69,;2.47,-4.02,;4.01,-4.02,;4.78,-2.69,;6.31,-2.69,;7.09,-1.36,;8.62,-1.36,;9.4,-.03,;8.62,1.3,;7.09,1.3,;6.31,-.03,;9.4,2.65,;10.93,2.65,;8.62,3.98,;9.4,5.33,;8.62,6.66,;7.09,6.66,;6.31,5.29,;7.09,3.98,;4.01,-1.35,;2.47,-1.36,;-5.18,.16,;-6.51,.98,)| Show InChI InChI=1S/C38H36N4/c1-39(35-9-5-3-6-10-35)37-21-25-41(26-22-37)29-31-13-17-33(18-14-31)34-19-15-32(16-20-34)30-42-27-23-38(24-28-42)40(2)36-11-7-4-8-12-36/h3-28H,29-30H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154643
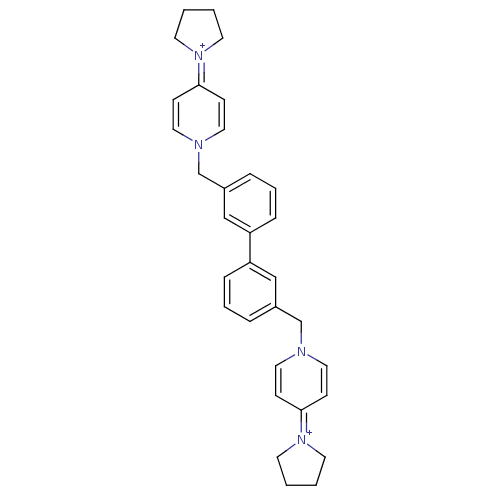
(4-tetrahydro-1H-1-pyrrolyl-1-{3-[3-(4-tetrahydro-1...)Show SMILES C(c1cccc(c1)-c1cccc(Cn2ccc(cc2)=[N+]2CCCC2)c1)n1ccc(cc1)=[N+]1CCCC1 |(-4.02,.24,;-3.24,-1.11,;-4,-2.43,;-3.24,-3.77,;-1.7,-3.77,;-.93,-2.43,;-1.7,-1.11,;.62,-2.43,;1.38,-3.77,;2.93,-3.77,;3.71,-2.43,;2.93,-1.09,;3.69,.26,;5.22,.26,;6,-1.08,;7.54,-1.08,;8.31,.29,;7.53,1.61,;5.98,1.59,;9.86,.29,;10.75,-.97,;12.22,-.47,;12.2,1.07,;10.75,1.54,;1.38,-1.11,;-5.55,.21,;-6.92,-.5,;-8.22,.32,;-8.17,1.86,;-6.82,2.57,;-5.5,1.76,;-9.5,2.69,;-9.58,4.22,;-11.07,4.59,;-11.88,3.28,;-10.91,2.11,)| Show InChI InChI=1S/C32H36N4/c1-2-16-35(15-1)31-11-19-33(20-12-31)25-27-7-5-9-29(23-27)30-10-6-8-28(24-30)26-34-21-13-32(14-22-34)36-17-3-4-18-36/h5-14,19-24H,1-4,15-18,25-26H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154650

(4N-(4-chlorophenyl)-4N-methyl-1-(3-{4-[4-chloro(me...)Show SMILES C[N+](c1ccc(Cl)cc1)=c1ccn(Cc2cccc(Cn3ccc(cc3)=[N+](C)c3ccc(Cl)cc3)c2)cc1 |(1.31,2.77,;2.64,2,;3.97,2.78,;3.97,4.31,;5.3,5.08,;6.63,4.32,;7.98,5.09,;6.65,2.78,;5.33,2,;2.66,.47,;3.97,-.29,;3.97,-1.83,;2.66,-2.6,;2.68,-4.14,;1.34,-4.91,;1.34,-6.45,;,-7.23,;-1.32,-6.45,;-1.32,-4.92,;-2.65,-4.14,;-2.65,-2.6,;-3.99,-1.83,;-3.99,-.29,;-2.65,.49,;-1.32,-.29,;-1.31,-1.83,;-2.66,2.02,;-1.35,2.8,;-4.01,2.77,;-4.01,4.31,;-5.34,5.06,;-6.65,4.27,;-8,5.06,;-6.63,2.75,;-5.31,2,;,-4.14,;1.34,-1.85,;1.33,-.29,)| Show InChI InChI=1S/C32H30Cl2N4/c1-35(29-10-6-27(33)7-11-29)31-14-18-37(19-15-31)23-25-4-3-5-26(22-25)24-38-20-16-32(17-21-38)36(2)30-12-8-28(34)9-13-30/h3-22H,23-24H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154662
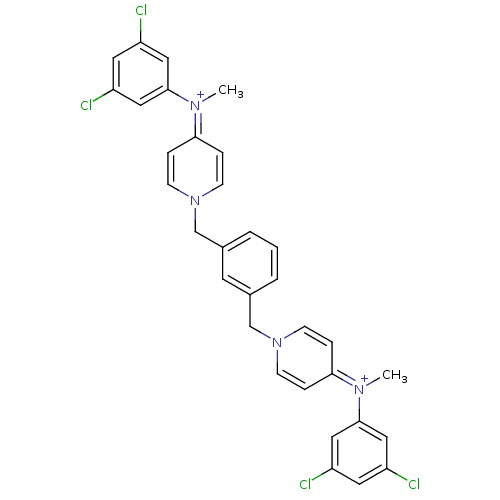
(4N-(3,5-dichlorophenyl)-4N-methyl-1-(3-{4-[3,5-dic...)Show SMILES C[N+](c1cc(Cl)cc(Cl)c1)=c1ccn(Cc2cccc(Cn3ccc(cc3)=[N+](C)c3cc(Cl)cc(Cl)c3)c2)cc1 |(-1.32,2.65,;-2.65,1.88,;-3.99,2.63,;-3.99,4.17,;-5.31,4.92,;-5.34,6.43,;-6.64,4.15,;-6.62,2.61,;-7.95,1.81,;-5.3,1.86,;-2.65,.34,;-1.31,-.43,;-1.31,-1.97,;-2.63,-2.74,;-2.63,-4.28,;-1.31,-5.05,;-1.31,-6.59,;.03,-7.36,;1.35,-6.59,;1.35,-5.05,;2.68,-4.28,;2.68,-2.73,;4.02,-1.97,;4.02,-.43,;2.68,.34,;1.34,-.44,;1.34,-1.98,;2.66,1.87,;1.34,2.63,;3.97,2.65,;3.97,4.17,;5.3,4.94,;5.3,6.46,;6.65,4.19,;6.67,2.65,;8.01,1.88,;5.33,1.87,;.03,-4.28,;-3.97,-1.97,;-3.97,-.43,)| Show InChI InChI=1S/C32H28Cl4N4/c1-37(31-17-25(33)15-26(34)18-31)29-6-10-39(11-7-29)21-23-4-3-5-24(14-23)22-40-12-8-30(9-13-40)38(2)32-19-27(35)16-28(36)20-32/h3-20H,21-22H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154642
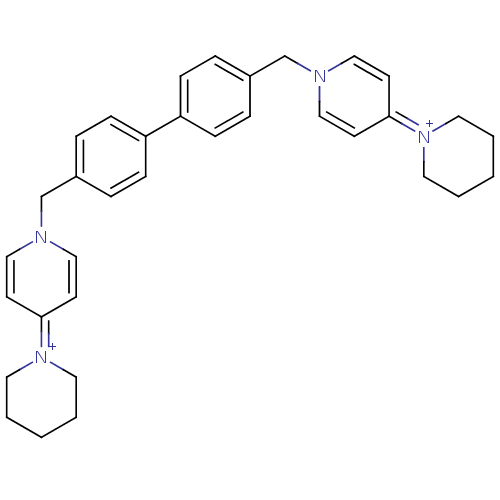
(4-hexahydro-1-pyridinyl-1-{4-[4-(4-hexahydro-1-pyr...)Show SMILES C(c1ccc(cc1)-c1ccc(Cn2ccc(cc2)=[N+]2CCCCC2)cc1)n1ccc(cc1)=[N+]1CCCCC1 |(-4.88,-2.66,;-3.33,-2.66,;-2.58,-1.33,;-1.03,-1.33,;-.26,-2.66,;-1.03,-3.99,;-2.58,-3.99,;1.28,-2.66,;2.05,-1.33,;3.58,-1.32,;4.37,-2.66,;5.91,-2.66,;6.68,-1.33,;8.21,-1.33,;8.99,,;8.21,1.34,;6.65,1.34,;5.91,,;8.98,2.68,;10.51,2.68,;11.28,4.01,;10.51,5.34,;8.98,5.34,;8.21,4.01,;3.58,-3.99,;2.05,-3.99,;-5.66,-1.33,;-7.02,-2.05,;-8.34,-1.23,;-8.29,.29,;-6.93,1.02,;-5.61,.21,;-9.59,1.1,;-9.55,2.65,;-10.86,3.47,;-12.19,2.75,;-12.26,1.2,;-10.95,.38,)| Show InChI InChI=1S/C34H40N4/c1-3-19-37(20-4-1)33-15-23-35(24-16-33)27-29-7-11-31(12-8-29)32-13-9-30(10-14-32)28-36-25-17-34(18-26-36)38-21-5-2-6-22-38/h7-18,23-26H,1-6,19-22,27-28H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154663

(4N-(3,5-dichlorophenyl)-4N-methyl-1-(4-{4-[3,5-dic...)Show SMILES C[N+](c1cc(Cl)cc(Cl)c1)=c1ccn(Cc2ccc(Cn3ccc(cc3)=[N+](C)c3cc(Cl)cc(Cl)c3)cc2)cc1 |(-6.66,2.21,;-6.68,.67,;-8.01,-.08,;-9.32,.72,;-10.65,-.03,;-11.97,.74,;-10.68,-1.57,;-9.37,-2.35,;-9.37,-3.89,;-8.04,-1.6,;-5.35,-.12,;-5.39,-1.67,;-4.06,-2.46,;-2.7,-1.71,;-1.93,-3.05,;-.39,-3.05,;.38,-4.37,;1.93,-4.37,;2.7,-3.04,;4.24,-3.04,;4.99,-1.71,;3.66,-.97,;3.66,.58,;4.99,1.37,;6.32,.6,;6.32,-.94,;4.99,2.89,;3.63,3.67,;6.32,3.69,;6.3,5.21,;7.63,5.98,;7.63,7.51,;8.99,5.23,;8.99,3.69,;10.32,2.91,;7.65,2.89,;1.93,-1.71,;.38,-1.71,;-2.68,-.17,;-4.01,.62,)| Show InChI InChI=1S/C32H28Cl4N4/c1-37(31-17-25(33)15-26(34)18-31)29-7-11-39(12-8-29)21-23-3-5-24(6-4-23)22-40-13-9-30(10-14-40)38(2)32-19-27(35)16-28(36)20-32/h3-20H,21-22H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154660
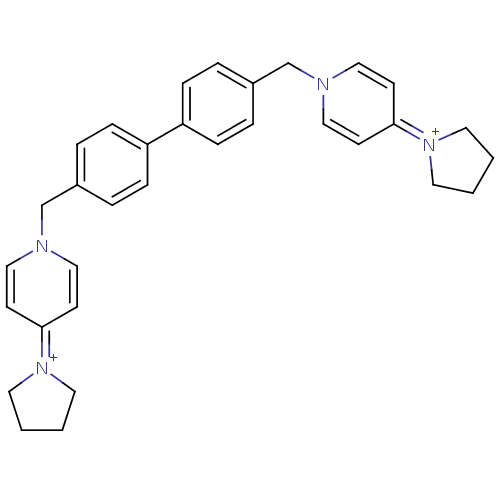
(4-tetrahydro-1H-1-pyrrolyl-1-{4-[4-(4-tetrahydro-1...)Show SMILES C(c1ccc(cc1)-c1ccc(Cn2ccc(cc2)=[N+]2CCCC2)cc1)n1ccc(cc1)=[N+]1CCCC1 |(-4.9,-2.46,;-3.36,-2.46,;-2.61,-1.12,;-1.07,-1.12,;-.3,-2.46,;-1.07,-3.79,;-2.61,-3.79,;1.24,-2.46,;2.01,-1.12,;3.55,-1.12,;4.32,-2.46,;5.86,-2.46,;6.63,-1.12,;8.17,-1.12,;8.92,.2,;8.17,1.53,;6.6,1.53,;5.86,.2,;8.92,2.89,;10.45,3.03,;10.76,4.54,;9.43,5.31,;8.28,4.27,;3.55,-3.79,;2.01,-3.79,;-5.69,-1.12,;-7.04,-1.85,;-8.35,-1.03,;-8.32,.49,;-6.95,1.21,;-5.64,.4,;-9.63,1.31,;-9.72,2.82,;-11.21,3.19,;-12.02,1.88,;-11.05,.72,)| Show InChI InChI=1S/C32H36N4/c1-2-18-35(17-1)31-13-21-33(22-14-31)25-27-5-9-29(10-6-27)30-11-7-28(8-12-30)26-34-23-15-32(16-24-34)36-19-3-4-20-36/h5-16,21-24H,1-4,17-20,25-26H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154647

(4N-methyl-4N-phenyl-1-[4-(4-methylanilino-1-pyridi...)Show SMILES C[N+](c1ccccc1)=c1ccn(Cc2ccc(Cn3ccc(cc3)=[N+](C)c3ccccc3)cc2)cc1 |(-7.75,-.52,;-6.38,.2,;-6.35,1.74,;-7.66,2.55,;-7.66,4.08,;-6.29,4.83,;-4.97,4.03,;-4.99,2.49,;-5.06,-.59,;-3.72,.16,;-2.38,-.64,;-2.42,-2.18,;-1.65,-3.51,;-.11,-3.51,;.66,-2.18,;2.2,-2.16,;2.98,-3.49,;4.52,-3.49,;5.28,-2.16,;3.93,-1.41,;3.93,.13,;5.27,.9,;6.61,.13,;6.61,-1.41,;5.27,2.45,;6.6,3.22,;3.93,3.19,;2.64,2.43,;1.32,3.18,;1.31,4.71,;2.62,5.48,;3.95,4.71,;2.2,-4.82,;.66,-4.82,;-3.78,-2.93,;-5.09,-2.11,)| Show InChI InChI=1S/C32H32N4/c1-33(29-9-5-3-6-10-29)31-17-21-35(22-18-31)25-27-13-15-28(16-14-27)26-36-23-19-32(20-24-36)34(2)30-11-7-4-8-12-30/h3-24H,25-26H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154652
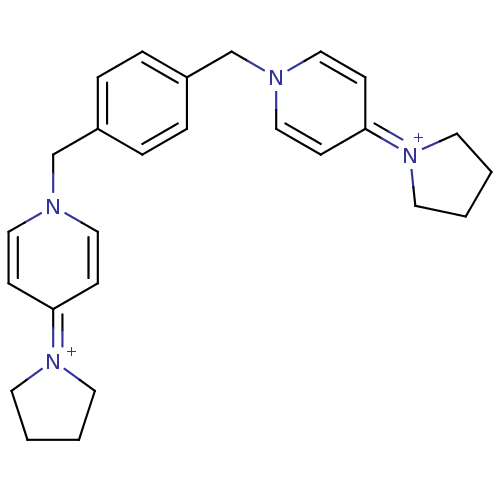
(4-tetrahydro-1H-1-pyrrolyl-1-[4-(4-tetrahydro-1H-1...)Show SMILES C(c1ccc(Cn2ccc(cc2)=[N+]2CCCC2)cc1)n1ccc(cc1)=[N+]1CCCC1 |(4.09,-2.71,;2.55,-2.71,;1.78,-4.04,;.24,-4.04,;-.53,-2.71,;-2.05,-2.71,;-2.84,-1.38,;-2.79,.16,;-4.12,.96,;-5.48,.21,;-5.5,-1.33,;-4.19,-2.12,;-6.79,1,;-6.91,2.54,;-8.4,2.89,;-9.21,1.56,;-8.21,.41,;.24,-1.38,;1.78,-1.38,;4.86,-1.38,;3.51,-.63,;3.51,.91,;4.86,1.7,;6.19,.93,;6.19,-.61,;4.86,3.22,;6.08,4.13,;5.6,5.58,;4.07,5.58,;3.6,4.12,)| Show InChI InChI=1S/C26H32N4/c1-2-14-29(13-1)25-9-17-27(18-10-25)21-23-5-7-24(8-6-23)22-28-19-11-26(12-20-28)30-15-3-4-16-30/h5-12,17-20H,1-4,13-16,21-22H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154655

(4-hexahydro-1-pyridinyl-1-[3-(4-hexahydro-1-pyridi...)Show SMILES C[N+](c1ccccc1)=c1ccn(Cc2cccc(Cn3ccc(cc3)=[N+](C)c3ccccc3)c2)cc1 |(1.31,3.04,;2.65,2.28,;3.98,3.06,;3.97,4.6,;5.31,5.35,;6.65,4.6,;6.66,3.06,;5.34,2.28,;2.67,.74,;3.98,-.02,;3.98,-1.57,;2.67,-2.32,;2.69,-3.88,;1.34,-4.64,;1.34,-6.2,;,-6.98,;-1.32,-6.2,;-1.32,-4.66,;-2.66,-3.89,;-2.66,-2.32,;-1.31,-1.57,;-1.32,-.02,;-2.66,.75,;-4,-.02,;-4,-1.57,;-2.67,2.29,;-1.35,3.08,;-4.01,3.04,;-4.01,4.6,;-5.35,5.32,;-6.66,4.55,;-6.64,3.03,;-5.32,2.28,;,-3.88,;1.34,-1.57,;1.33,-.03,)| Show InChI InChI=1S/C32H32N4/c1-33(29-12-5-3-6-13-29)31-16-20-35(21-17-31)25-27-10-9-11-28(24-27)26-36-22-18-32(19-23-36)34(2)30-14-7-4-8-15-30/h3-24H,25-26H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154640
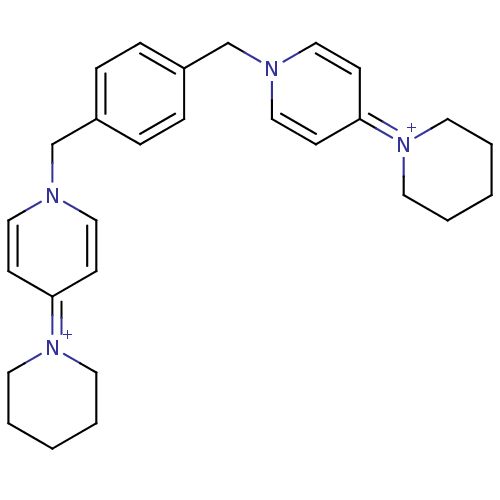
(4-hexahydro-1-pyridinyl-1-[4-(4-hexahydro-1-pyridi...)Show SMILES C(c1ccc(Cn2ccc(cc2)=[N+]2CCCCC2)cc1)n1ccc(cc1)=[N+]1CCCCC1 |(4.21,-2.95,;2.68,-2.95,;1.91,-4.28,;.37,-4.28,;-.39,-2.95,;-1.93,-2.95,;-2.71,-1.62,;-2.68,-.1,;-4.01,.72,;-5.34,-.03,;-5.38,-1.57,;-4.05,-2.36,;-6.67,.76,;-6.64,2.3,;-7.95,3.1,;-9.3,2.37,;-9.33,.83,;-8.02,.02,;.37,-1.62,;1.91,-1.62,;4.98,-1.62,;3.65,-.87,;3.64,.67,;4.98,1.45,;6.31,.69,;6.31,-.85,;4.97,2.98,;6.31,3.75,;6.3,5.27,;4.98,6.06,;3.65,5.27,;3.65,3.73,)| Show InChI InChI=1S/C28H36N4/c1-3-15-31(16-4-1)27-11-19-29(20-12-27)23-25-7-9-26(10-8-25)24-30-21-13-28(14-22-30)32-17-5-2-6-18-32/h7-14,19-22H,1-6,15-18,23-24H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154661

(4N-(4-chlorophenyl)-4N-methyl-1-[2-{4-[4-chloro(me...)Show SMILES C[N+](c1ccc(Cl)cc1)=c1ccn(CC2CC2Cn2ccc(cc2)=[N+](C)c2ccc(Cl)cc2)cc1 |(-.77,2.3,;-2.1,1.53,;-3.45,2.3,;-4.76,1.53,;-6.11,2.28,;-6.11,3.82,;-7.44,4.59,;-4.78,4.61,;-3.45,3.84,;-2.09,-.01,;-3.42,-.78,;-3.42,-2.32,;-2.09,-3.09,;-2.09,-4.63,;-.75,-5.4,;.03,-6.73,;.78,-5.4,;2.12,-4.6,;2.12,-3.06,;3.45,-2.31,;3.45,-.78,;2.11,-.01,;.78,-.78,;.78,-2.32,;2.11,1.53,;.77,2.3,;3.43,2.3,;4.77,1.53,;6.1,2.3,;6.1,3.84,;7.43,4.61,;4.74,4.61,;3.43,3.82,;-.76,-2.32,;-.76,-.78,)| Show InChI InChI=1S/C29H30Cl2N4/c1-32(26-7-3-24(30)4-8-26)28-11-15-34(16-12-28)20-22-19-23(22)21-35-17-13-29(14-18-35)33(2)27-9-5-25(31)6-10-27/h3-18,22-23H,19-21H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154649
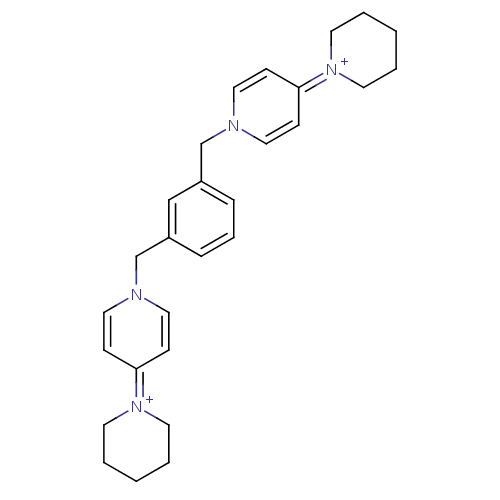
(4-hexahydro-1-pyridinyl-1-[2-(4-hexahydro-1-pyridi...)Show SMILES C(c1cccc(Cn2ccc(cc2)=[N+]2CCCCC2)c1)n1ccc(cc1)=[N+]1CCCCC1 |(-2.65,-3.58,;-1.33,-4.34,;-1.33,-5.89,;,-6.65,;1.34,-5.89,;1.34,-4.32,;2.68,-3.55,;2.66,-2.01,;3.97,-1.24,;3.97,.3,;2.66,1.05,;1.31,.27,;1.34,-1.27,;2.64,2.58,;3.97,3.35,;3.97,4.89,;2.66,5.64,;1.34,4.89,;1.34,3.33,;,-3.55,;-2.65,-2.01,;-1.31,-1.24,;-1.33,.3,;-2.65,1.07,;-3.99,.3,;-3.99,-1.24,;-2.66,2.61,;-1.33,3.36,;-1.35,4.89,;-2.66,5.65,;-4.01,4.89,;-4.01,3.35,)| Show InChI InChI=1S/C28H36N4/c1-3-14-31(15-4-1)27-10-18-29(19-11-27)23-25-8-7-9-26(22-25)24-30-20-12-28(13-21-30)32-16-5-2-6-17-32/h7-13,18-22H,1-6,14-17,23-24H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154645
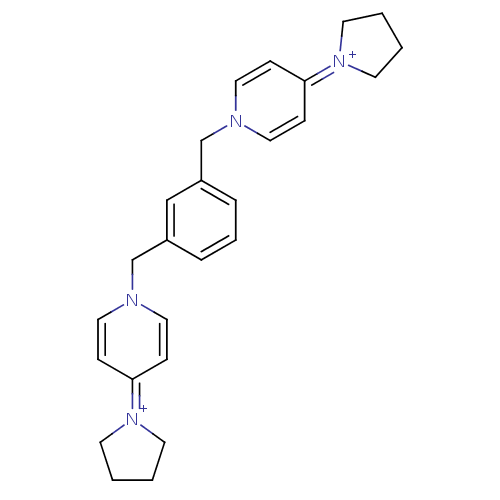
(4-tetrahydro-1H-1-pyrrolyl-1-[3-(4-tetrahydro-1H-1...)Show SMILES C(c1cccc(Cn2ccc(cc2)=[N+]2CCCC2)c1)n1ccc(cc1)=[N+]1CCCC1 |(-2.65,-3.2,;-1.32,-3.97,;-1.32,-5.51,;.01,-6.28,;1.34,-5.51,;1.34,-3.97,;2.68,-3.2,;2.67,-1.66,;4,-.89,;4,.65,;2.64,1.42,;1.33,.65,;1.33,-.91,;2.64,2.96,;3.88,3.87,;3.41,5.31,;1.87,5.31,;1.38,3.85,;.01,-3.2,;-2.65,-1.66,;-1.31,-.89,;-1.32,.65,;-2.66,1.42,;-4.01,.65,;-4.01,-.89,;-2.66,2.96,;-3.91,3.87,;-3.45,5.32,;-1.91,5.34,;-1.42,3.87,)| Show InChI InChI=1S/C26H32N4/c1-2-13-29(12-1)25-8-16-27(17-9-25)21-23-6-5-7-24(20-23)22-28-18-10-26(11-19-28)30-14-3-4-15-30/h5-11,16-20H,1-4,12-15,21-22H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154657

(4-hexahydro-1-pyridinyl-1-[2-(4-hexahydro-1-pyridi...)Show SMILES C(C1CC1Cn1ccc(cc1)=[N+]1CCCCC1)n1ccc(cc1)=[N+]1CCCCC1 |(-2.09,-4.11,;-.75,-4.88,;.03,-6.21,;.79,-4.86,;2.12,-4.09,;2.12,-2.55,;3.45,-1.78,;3.45,-.24,;2.11,.51,;.78,-.26,;.78,-1.8,;2.11,2.05,;3.43,2.82,;3.43,4.33,;2.11,5.11,;.78,4.33,;.78,2.8,;-2.09,-2.57,;-3.42,-1.8,;-3.42,-.24,;-2.09,.53,;-.76,-.24,;-.76,-1.8,;-2.1,2.05,;-.77,2.84,;-.77,4.36,;-2.1,5.13,;-3.45,4.36,;-3.45,2.82,)| Show InChI InChI=1S/C25H36N4/c1-3-11-28(12-4-1)24-7-15-26(16-8-24)20-22-19-23(22)21-27-17-9-25(10-18-27)29-13-5-2-6-14-29/h7-10,15-18,22-23H,1-6,11-14,19-21H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154648

(4N-methyl-4N-phenyl-1-[2-(4-methylanilino-1-pyridi...)Show SMILES C[N+](c1ccccc1)=c1ccn(CC2CC2Cn2ccc(cc2)=[N+](C)c2ccccc2)cc1 |(-.79,2.58,;-2.1,1.81,;-3.45,2.58,;-3.45,4.12,;-4.78,4.89,;-6.11,4.12,;-6.09,2.56,;-4.78,1.81,;-2.09,.27,;-3.42,-.5,;-3.42,-2.04,;-2.09,-2.81,;-2.09,-4.35,;-.75,-5.12,;.03,-6.45,;.78,-5.11,;2.13,-4.32,;2.12,-2.78,;3.44,-2.04,;3.44,-.5,;2.12,.27,;.78,-.5,;.78,-2.04,;2.11,1.81,;.77,2.58,;3.44,2.58,;4.77,1.81,;6.11,2.58,;6.1,4.12,;4.74,4.89,;3.43,4.12,;-.75,-2.04,;-.76,-.5,)| Show InChI InChI=1S/C29H32N4/c1-30(26-9-5-3-6-10-26)28-13-17-32(18-14-28)22-24-21-25(24)23-33-19-15-29(16-20-33)31(2)27-11-7-4-8-12-27/h3-20,24-25H,21-23H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154659
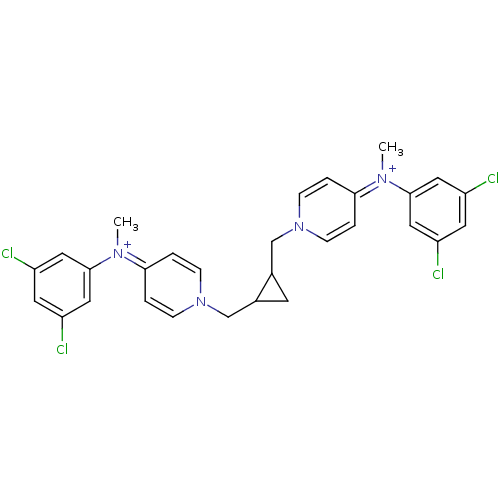
(4N-(3,5-dichlorophenyl)-4N-methyl-1-[2-{4-[3,5-dic...)Show SMILES C[N+](c1cc(Cl)cc(Cl)c1)=c1ccn(CC2CC2Cn2ccc(cc2)=[N+](C)c2cc(Cl)cc(Cl)c2)cc1 |(.78,2.12,;2.12,1.35,;3.45,2.12,;4.79,1.35,;6.12,2.12,;7.45,1.35,;6.11,3.66,;4.77,4.43,;4.76,5.97,;3.43,3.63,;2.12,-.19,;3.46,-.96,;3.46,-2.5,;2.12,-3.25,;2.15,-4.79,;.8,-5.58,;.05,-6.91,;-.74,-5.58,;-2.08,-4.81,;-2.08,-3.27,;-.74,-2.5,;-.75,-.96,;-2.08,-.17,;-3.41,-.96,;-3.41,-2.5,;-2.09,1.35,;-.76,2.12,;-3.42,2.12,;-3.42,3.66,;-4.76,4.43,;-4.78,5.97,;-6.09,3.63,;-6.09,2.09,;-7.41,1.32,;-4.75,1.35,;.8,-2.5,;.79,-.96,)| Show InChI InChI=1S/C29H28Cl4N4/c1-34(28-14-22(30)12-23(31)15-28)26-3-7-36(8-4-26)18-20-11-21(20)19-37-9-5-27(6-10-37)35(2)29-16-24(32)13-25(33)17-29/h3-10,12-17,20-21H,11,18-19H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50154641

(4-tetrahydro-1H-1-pyrrolyl-1-[2-(4-tetrahydro-1H-1...)Show SMILES C(C1CC1Cn1ccc(cc1)=[N+]1CCCC1)n1ccc(cc1)=[N+]1CCCC1 |(-2.12,-3.79,;-.75,-4.56,;.03,-5.89,;.78,-4.56,;2.13,-3.76,;2.11,-2.22,;3.44,-1.48,;3.44,.06,;2.11,.83,;.78,.06,;.78,-1.48,;2.11,2.37,;3.36,3.28,;2.85,4.73,;1.31,4.73,;.85,3.26,;-2.12,-2.25,;-3.42,-1.48,;-3.42,.06,;-2.12,.83,;-.76,.06,;-.75,-1.48,;-2.12,2.37,;-3.35,3.28,;-2.89,4.75,;-1.35,4.75,;-.86,3.28,)| Show InChI InChI=1S/C23H32N4/c1-2-10-26(9-1)22-5-13-24(14-6-22)18-20-17-21(20)19-25-15-7-23(8-16-25)27-11-3-4-12-27/h5-8,13-16,20-21H,1-4,9-12,17-19H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human choline kinase enzyme |
J Med Chem 47: 5433-40 (2004)
Article DOI: 10.1021/jm0496537
BindingDB Entry DOI: 10.7270/Q22V2FM2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data