 Found 41 hits Enz. Inhib. hit(s) with all data for entry = 50016194
Found 41 hits Enz. Inhib. hit(s) with all data for entry = 50016194 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166208

(Bisquinolinium derivative | CHEMBL191595)Show SMILES C\[N+](c1ccccc1)=c1\ccn(Cc2cccc(c2)-c2cccc(Cn3cc\c(=[N+](/C)c4ccccc4)c4ccccc34)c2)c2ccccc12 Show InChI InChI=1S/C46H40N4/c1-47(39-19-5-3-6-20-39)43-27-29-49(45-25-11-9-23-41(43)45)33-35-15-13-17-37(31-35)38-18-14-16-36(32-38)34-50-30-28-44(42-24-10-12-26-46(42)50)48(2)40-21-7-4-8-22-40/h3-32H,33-34H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166185
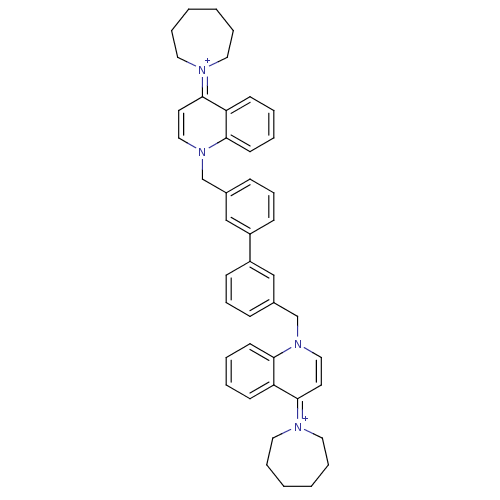
(Bisquinolinium derivative | CHEMBL372667)Show SMILES C(c1cccc(c1)-c1cccc(Cn2ccc(=[N+]3CCCCCC3)c3ccccc23)c1)n1ccc(=[N+]2CCCCCC2)c2ccccc12 |(-4.01,-2.08,;-2.68,-2.85,;-3.45,-4.18,;-2.7,-5.51,;-1.16,-5.53,;-.37,-4.18,;-1.14,-2.85,;1.15,-4.18,;1.92,-5.51,;3.46,-5.51,;4.23,-4.18,;3.46,-2.85,;4.74,-2.01,;4.67,-.47,;3.31,.23,;3.25,1.79,;4.55,2.61,;4.48,4.15,;5.84,4.88,;6.11,6.41,;5.09,7.54,;3.55,7.47,;2.64,6.25,;3.06,4.75,;5.91,1.89,;7.21,2.72,;8.57,2.01,;8.64,.47,;7.35,-.36,;5.98,.35,;1.92,-2.85,;-4.01,-.54,;-2.68,.23,;-2.7,1.77,;-4.03,2.54,;-4.05,4.08,;-2.66,4.76,;-2.35,6.27,;-3.33,7.46,;-4.85,7.44,;-5.8,6.22,;-5.43,4.73,;-5.36,1.77,;-6.69,2.52,;-8.02,1.75,;-8.02,.2,;-6.69,-.57,;-5.36,.21,)| Show InChI InChI=1S/C44H48N4/c1-2-10-26-45(25-9-1)43-23-29-47(41-21-7-5-19-39(41)43)33-35-15-13-17-37(31-35)38-18-14-16-36(32-38)34-48-30-24-44(40-20-6-8-22-42(40)48)46-27-11-3-4-12-28-46/h5-8,13-24,29-32H,1-4,9-12,25-28,33-34H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166194

(Bisquinolinium derivative | CHEMBL363853)Show SMILES C(Cc1ccc(Cn2ccc(=[N+]3CCCCCC3)c3ccccc23)cc1)c1ccc(Cn2ccc(=[N+]3CCCCCC3)c3ccccc23)cc1 |(3.57,-5.32,;2.82,-3.97,;1.28,-3.94,;.54,-2.6,;-1,-2.57,;-1.8,-3.87,;-3.21,-3.27,;-3.4,-1.73,;-2.16,-.82,;-2.35,.72,;-3.76,1.32,;-3.96,2.86,;-5.4,3.35,;-5.94,4.82,;-5.15,6.13,;-3.61,6.31,;-2.5,5.24,;-2.65,3.7,;-4.98,.41,;-6.41,1,;-7.64,.09,;-7.46,-1.45,;-6.04,-2.06,;-4.81,-1.13,;-1.07,-5.23,;.47,-5.26,;5.11,-5.35,;5.84,-6.7,;7.38,-6.73,;8.17,-5.41,;9.49,-4.62,;9.46,-3.08,;8.1,-2.34,;8.07,-.78,;9.39,.02,;9.34,1.56,;7.95,2.19,;7.57,3.68,;8.49,4.91,;10.03,4.95,;11.02,3.77,;10.73,2.26,;10.73,-.73,;12.05,.06,;13.4,-.66,;13.44,-2.22,;12.12,-3.02,;10.76,-2.27,;7.43,-4.06,;5.89,-4.04,)| Show InChI InChI=1S/C46H52N4/c1-2-10-30-47(29-9-1)45-27-33-49(43-15-7-5-13-41(43)45)35-39-23-19-37(20-24-39)17-18-38-21-25-40(26-22-38)36-50-34-28-46(42-14-6-8-16-44(42)50)48-31-11-3-4-12-32-48/h5-8,13-16,19-28,33-34H,1-4,9-12,17-18,29-32,35-36H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166193

(Bisquinolinium derivative | CHEMBL370289)Show SMILES Clc1ccc2c(c1)n(Cc1ccc(CCc3ccc(Cn4ccc(=[N+]5CCCC5)c5ccc(Cl)cc45)cc3)cc1)ccc2=[N+]1CCCC1 |(18.29,-4.32,;16.94,-3.58,;16.89,-2.04,;15.54,-1.31,;14.23,-2.11,;14.27,-3.65,;15.63,-4.39,;12.95,-4.44,;12.99,-5.98,;11.67,-6.79,;10.92,-5.43,;9.38,-5.41,;8.61,-6.72,;7.07,-6.69,;6.33,-5.35,;4.79,-5.3,;4.04,-3.96,;2.52,-3.95,;1.71,-5.25,;.35,-4.52,;.29,-2.99,;1.59,-2.17,;1.52,-.61,;.17,.09,;.1,1.62,;-1.18,2.47,;-.76,3.96,;.78,4.03,;1.31,2.58,;-1.13,-.73,;-2.49,-.01,;-3.79,-.82,;-3.75,-2.36,;-5.05,-3.18,;-2.38,-3.09,;-1.07,-2.27,;2.43,-6.6,;3.97,-6.63,;9.34,-8.05,;10.88,-8.1,;11.6,-3.71,;11.58,-2.15,;12.88,-1.36,;12.85,.18,;14.07,1.1,;13.56,2.56,;12.02,2.52,;11.58,1.04,)| Show InChI InChI=1S/C42H42Cl2N4/c43-35-15-17-37-39(45-21-1-2-22-45)19-25-47(41(37)27-35)29-33-11-7-31(8-12-33)5-6-32-9-13-34(14-10-32)30-48-26-20-40(46-23-3-4-24-46)38-18-16-36(44)28-42(38)48/h7-20,25-28H,1-6,21-24,29-30H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50054098
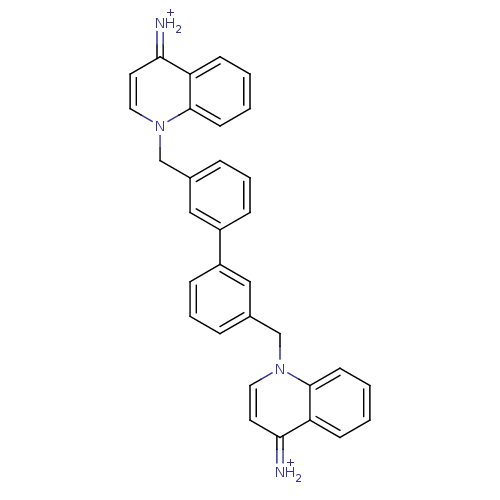
(1,1'-[biphenyl-3,3'-diylbis(methylene)]-bis(4-amin...)Show SMILES [NH2+]=c1ccn(Cc2cccc(c2)-c2cccc(Cn3ccc(=[NH2+])c4ccccc34)c2)c2ccccc12 Show InChI InChI=1S/C32H26N4/c33-29-15-17-35(31-13-3-1-11-27(29)31)21-23-7-5-9-25(19-23)26-10-6-8-24(20-26)22-36-18-16-30(34)28-12-2-4-14-32(28)36/h1-20,33-34H,21-22H2/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166183

(Bisquinolinium derivative | CHEMBL189324)Show SMILES Clc1ccc2c(c1)n(Cc1cccc(c1)-c1cccc(Cn3ccc(=[N+]4CCCC4)c4ccc(Cl)cc34)c1)ccc2=[N+]1CCCC1 |(-7.92,.07,;-6.62,.89,;-6.66,2.43,;-5.36,3.24,;-4,2.53,;-3.94,.99,;-5.25,.16,;-2.58,.27,;-2.52,-1.27,;-1.16,-1.99,;-1.97,-3.29,;-1.24,-4.64,;.3,-4.67,;1.1,-3.36,;.36,-2.01,;2.64,-3.38,;3.38,-4.73,;4.92,-4.77,;5.72,-3.44,;4.97,-2.1,;6.28,-1.29,;6.23,.25,;4.88,.98,;4.86,2.53,;6.16,3.33,;6.13,4.87,;7.35,5.8,;6.84,7.27,;5.3,7.22,;4.86,5.75,;7.52,2.59,;8.82,3.38,;10.18,2.65,;10.22,1.11,;11.57,.37,;8.91,.3,;7.56,1.04,;3.44,-2.06,;-1.27,1.09,;-1.34,2.64,;-2.7,3.35,;-2.77,4.89,;-4.05,5.73,;-3.63,7.22,;-2.09,7.28,;-1.55,5.85,)| Show InChI InChI=1S/C40H38Cl2N4/c41-33-11-13-35-37(43-17-1-2-18-43)15-21-45(39(35)25-33)27-29-7-5-9-31(23-29)32-10-6-8-30(24-32)28-46-22-16-38(44-19-3-4-20-44)36-14-12-34(42)26-40(36)46/h5-16,21-26H,1-4,17-20,27-28H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166184

(Bisquinolinium derivative | CHEMBL191873)Show SMILES C(c1cccc(c1)-c1cccc(Cn2cc\c(=[NH+]/c3ccccc3)c3ccccc23)c1)n1cc\c(=[NH+]\c2ccccc2)c2ccccc12 Show InChI InChI=1S/C44H34N4/c1-3-17-37(18-4-1)45-41-25-27-47(43-23-9-7-21-39(41)43)31-33-13-11-15-35(29-33)36-16-12-14-34(30-36)32-48-28-26-42(40-22-8-10-24-44(40)48)46-38-19-5-2-6-20-38/h1-30H,31-32H2/p+2/b45-41-,46-42+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166175

(Bisquinolinium derivative | CHEMBL364023)Show SMILES C\[N+](c1ccccc1)=c1\ccn(Cc2ccc(CCc3ccc(Cn4cc\c(=[N+](/C)c5ccccc5)c5ccccc45)cc3)cc2)c2ccccc12 Show InChI InChI=1S/C48H44N4/c1-49(41-13-5-3-6-14-41)45-31-33-51(47-19-11-9-17-43(45)47)35-39-27-23-37(24-28-39)21-22-38-25-29-40(30-26-38)36-52-34-32-46(44-18-10-12-20-48(44)52)50(2)42-15-7-4-8-16-42/h3-20,23-34H,21-22,35-36H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166172

(Bisquinolinium derivative | CHEMBL192986)Show SMILES C\[N+](c1ccc(Cl)cc1)=c1\ccn(Cc2ccc(cc2)-c2ccc(Cn3cc\c(=[N+](/C)c4ccc(Cl)cc4)c4ccccc34)cc2)c2ccccc12 Show InChI InChI=1S/C46H38Cl2N4/c1-49(39-23-19-37(47)20-24-39)43-27-29-51(45-9-5-3-7-41(43)45)31-33-11-15-35(16-12-33)36-17-13-34(14-18-36)32-52-30-28-44(42-8-4-6-10-46(42)52)50(2)40-25-21-38(48)22-26-40/h3-30H,31-32H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166202
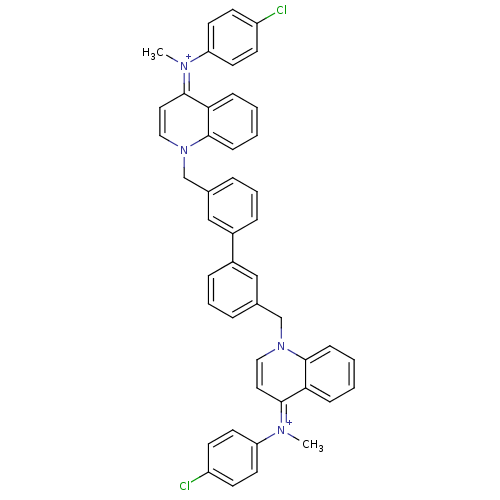
(Bisquinolinium derivative | CHEMBL372448)Show SMILES C\[N+](c1ccc(Cl)cc1)=c1\ccn(Cc2cccc(c2)-c2cccc(Cn3cc\c(=[N+](/C)c4ccc(Cl)cc4)c4ccccc34)c2)c2ccccc12 Show InChI InChI=1S/C46H38Cl2N4/c1-49(39-21-17-37(47)18-22-39)43-25-27-51(45-15-5-3-13-41(43)45)31-33-9-7-11-35(29-33)36-12-8-10-34(30-36)32-52-28-26-44(42-14-4-6-16-46(42)52)50(2)40-23-19-38(48)20-24-40/h3-30H,31-32H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166198

(Bisquinolinium derivative | CHEMBL366224)Show SMILES C(c1ccc(cc1)-c1ccc(Cn2ccc(=[N+]3CCCCCC3)c3ccccc23)cc1)n1ccc(=[N+]2CCCCCC2)c2ccccc12 |(-3.14,-3.12,;-1.73,-3.73,;-.99,-5.08,;.54,-5.11,;1.35,-3.8,;.61,-2.46,;-.92,-2.42,;2.88,-3.83,;3.67,-2.52,;5.21,-2.54,;5.95,-3.89,;7.27,-3.1,;7.24,-1.56,;5.88,-.82,;5.85,.74,;7.17,1.53,;7.12,3.07,;5.74,3.7,;5.35,5.19,;6.27,6.43,;7.81,6.46,;8.8,5.28,;8.51,3.77,;8.51,.78,;9.82,1.58,;11.18,.85,;11.21,-.7,;9.89,-1.5,;8.54,-.75,;5.16,-5.21,;3.62,-5.18,;-3.33,-1.59,;-2.09,-.68,;-2.28,.86,;-3.68,1.46,;-3.89,3,;-5.33,3.49,;-5.87,4.94,;-5.07,6.26,;-3.54,6.45,;-2.42,5.38,;-2.58,3.84,;-4.91,.55,;-6.33,1.14,;-7.55,.23,;-7.38,-1.31,;-5.97,-1.92,;-4.73,-.99,)| Show InChI InChI=1S/C44H48N4/c1-2-10-28-45(27-9-1)43-25-31-47(41-15-7-5-13-39(41)43)33-35-17-21-37(22-18-35)38-23-19-36(20-24-38)34-48-32-26-44(40-14-6-8-16-42(40)48)46-29-11-3-4-12-30-46/h5-8,13-26,31-32H,1-4,9-12,27-30,33-34H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166189
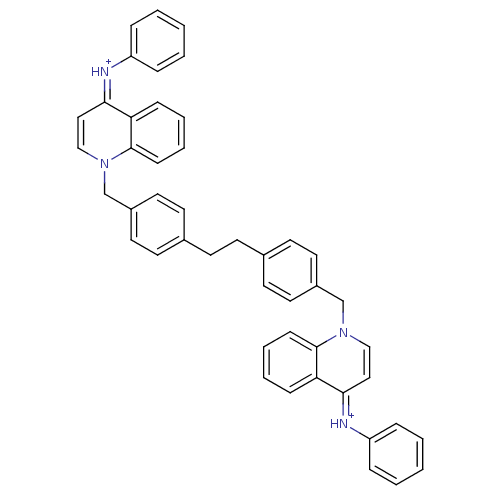
(Bisquinolinium derivative | CHEMBL364022)Show SMILES C(Cc1ccc(Cn2cc\c(=[NH+]/c3ccccc3)c3ccccc23)cc1)c1ccc(Cn2cc\c(=[NH+]\c3ccccc3)c3ccccc23)cc1 Show InChI InChI=1S/C46H38N4/c1-3-11-39(12-4-1)47-43-29-31-49(45-17-9-7-15-41(43)45)33-37-25-21-35(22-26-37)19-20-36-23-27-38(28-24-36)34-50-32-30-44(42-16-8-10-18-46(42)50)48-40-13-5-2-6-14-40/h1-18,21-32H,19-20,33-34H2/p+2/b47-43-,48-44+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166171

(Bisquinolinium derivative | CHEMBL436108)Show SMILES C\[N+](c1ccccc1)=c1\ccn(Cc2ccc(cc2)-c2ccc(Cn3cc\c(=[N+](/C)c4ccccc4)c4ccccc34)cc2)c2ccccc12 Show InChI InChI=1S/C46H40N4/c1-47(39-13-5-3-6-14-39)43-29-31-49(45-19-11-9-17-41(43)45)33-35-21-25-37(26-22-35)38-27-23-36(24-28-38)34-50-32-30-44(42-18-10-12-20-46(42)50)48(2)40-15-7-4-8-16-40/h3-32H,33-34H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166199

(Bisquinolinium derivative | CHEMBL364425)Show SMILES C\[N+](c1ccccc1)=c1\ccn(Cc2cccc(c2)-c2cccc(Cn3cc\c(=[N+](/C)c4ccccc4)c4ccc(Cl)cc34)c2)c2cc(Cl)ccc12 Show InChI InChI=1S/C46H38Cl2N4/c1-49(39-15-5-3-6-16-39)43-23-25-51(45-29-37(47)19-21-41(43)45)31-33-11-9-13-35(27-33)36-14-10-12-34(28-36)32-52-26-24-44(42-22-20-38(48)30-46(42)52)50(2)40-17-7-4-8-18-40/h3-30H,31-32H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166205

(Bisquinolinium derivative | CHEMBL193088)Show SMILES C\[N+](c1ccccc1)=c1\ccn(Cc2ccc(CCc3ccc(Cn4cc\c(=[N+](/C)c5ccccc5)c5ccc(Cl)cc45)cc3)cc2)c2cc(Cl)ccc12 Show InChI InChI=1S/C48H42Cl2N4/c1-51(41-9-5-3-6-10-41)45-27-29-53(47-31-39(49)23-25-43(45)47)33-37-19-15-35(16-20-37)13-14-36-17-21-38(22-18-36)34-54-30-28-46(44-26-24-40(50)32-48(44)54)52(2)42-11-7-4-8-12-42/h3-12,15-32H,13-14,33-34H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166210

(Bisquinolinium derivative | CHEMBL370712)Show SMILES C[N+](C)=c1ccn(Cc2cccc(c2)-c2cccc(Cn3ccc(=[N+](C)C)c4ccccc34)c2)c2ccccc12 |(5.78,5.2,;4.49,4.38,;3.12,5.09,;4.56,2.84,;3.26,2.03,;3.32,.46,;4.68,-.24,;4.75,-1.78,;3.47,-2.62,;4.24,-3.96,;3.47,-5.29,;1.92,-5.29,;1.15,-3.96,;1.92,-2.62,;-.37,-3.96,;-1.16,-5.3,;-2.7,-5.29,;-3.46,-3.96,;-2.68,-2.62,;-4.02,-1.85,;-4.02,-.31,;-2.69,.46,;-2.7,2,;-4.03,2.77,;-4.06,4.31,;-5.39,5.07,;-2.73,5.09,;-5.37,2,;-6.7,2.76,;-8.03,1.99,;-8.03,.44,;-6.7,-.34,;-5.37,.44,;-1.14,-2.62,;5.99,.58,;7.36,-.12,;8.65,.7,;8.58,2.24,;7.22,2.96,;5.92,2.13,)| Show InChI InChI=1S/C36H36N4/c1-37(2)33-19-21-39(35-17-7-5-15-31(33)35)25-27-11-9-13-29(23-27)30-14-10-12-28(24-30)26-40-22-20-34(38(3)4)32-16-6-8-18-36(32)40/h5-24H,25-26H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166174

(Bisquinolinium derivative | CHEMBL372223)Show SMILES C\[N+](c1ccc(Cl)cc1)=c1\ccn(Cc2ccc(CCc3ccc(Cn4cc\c(=[N+](/C)c5ccc(Cl)cc5)c5ccccc45)cc3)cc2)c2ccccc12 Show InChI InChI=1S/C48H42Cl2N4/c1-51(41-25-21-39(49)22-26-41)45-29-31-53(47-9-5-3-7-43(45)47)33-37-17-13-35(14-18-37)11-12-36-15-19-38(20-16-36)34-54-32-30-46(44-8-4-6-10-48(44)54)52(2)42-27-23-40(50)24-28-42/h3-10,13-32H,11-12,33-34H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166177

(Bisquinolinium derivative | CHEMBL364692)Show SMILES C\[N+](c1ccc(Cl)cc1)=c1\ccn(Cc2cccc(c2)-c2cccc(Cn3cc\c(=[N+](/C)c4ccc(Cl)cc4)c4ccc(Cl)cc34)c2)c2cc(Cl)ccc12 Show InChI InChI=1S/C46H36Cl4N4/c1-51(39-15-9-35(47)10-16-39)43-21-23-53(45-27-37(49)13-19-41(43)45)29-31-5-3-7-33(25-31)34-8-4-6-32(26-34)30-54-24-22-44(42-20-14-38(50)28-46(42)54)52(2)40-17-11-36(48)12-18-40/h3-28H,29-30H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166188
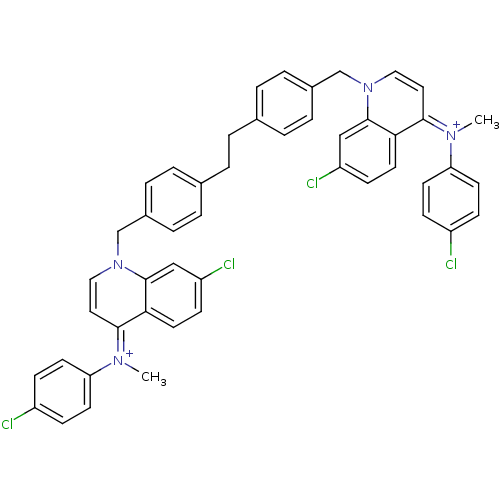
(Bisquinolinium derivative | CHEMBL192972)Show SMILES C\[N+](c1ccc(Cl)cc1)=c1\ccn(Cc2ccc(CCc3ccc(Cn4cc\c(=[N+](/C)c5ccc(Cl)cc5)c5ccc(Cl)cc45)cc3)cc2)c2cc(Cl)ccc12 Show InChI InChI=1S/C48H40Cl4N4/c1-53(41-19-13-37(49)14-20-41)45-25-27-55(47-29-39(51)17-23-43(45)47)31-35-9-5-33(6-10-35)3-4-34-7-11-36(12-8-34)32-56-28-26-46(44-24-18-40(52)30-48(44)56)54(2)42-21-15-38(50)16-22-42/h5-30H,3-4,31-32H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166195

(Bisquinolinium derivative | CHEMBL372473)Show SMILES C[N+](C)=c1ccn(Cc2ccc(CCc3ccc(Cn4ccc(=[N+](C)C)c5ccc(Cl)cc45)cc3)cc2)c2cc(Cl)ccc12 |(-.91,2.29,;.45,1.58,;1.76,2.39,;.52,.04,;1.87,-.68,;1.94,-2.22,;.64,-3.04,;.7,-4.58,;2.06,-5.3,;2.87,-4,;4.39,-4.02,;5.14,-5.36,;6.68,-5.4,;7.42,-6.75,;8.96,-6.77,;9.73,-5.47,;11.27,-5.49,;12.02,-6.84,;13.34,-6.04,;13.3,-4.5,;11.95,-3.76,;11.93,-2.22,;13.23,-1.42,;13.2,.12,;14.52,.93,;11.86,.86,;14.59,-2.15,;15.89,-1.36,;17.24,-2.1,;17.29,-3.64,;18.64,-4.37,;15.98,-4.44,;14.62,-3.69,;11.23,-8.16,;9.69,-8.12,;4.32,-6.69,;2.78,-6.66,;-.72,-2.32,;-2.03,-3.15,;-3.4,-2.43,;-4.7,-3.24,;-3.44,-.89,;-2.14,-.05,;-.79,-.78,)| Show InChI InChI=1S/C38H38Cl2N4/c1-41(2)35-19-21-43(37-23-31(39)15-17-33(35)37)25-29-11-7-27(8-12-29)5-6-28-9-13-30(14-10-28)26-44-22-20-36(42(3)4)34-18-16-32(40)24-38(34)44/h7-24H,5-6,25-26H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166180
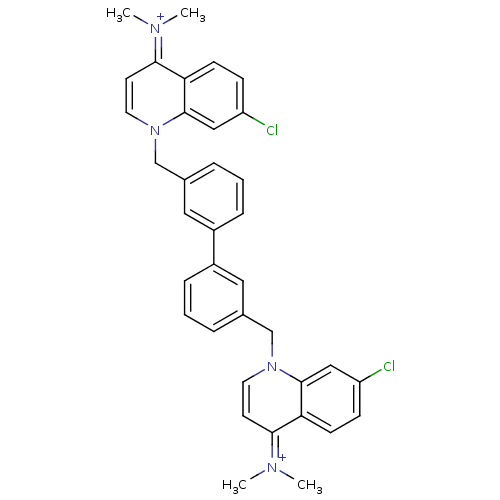
(Bisquinolinium derivative | CHEMBL193153)Show SMILES C[N+](C)=c1ccn(Cc2cccc(c2)-c2cccc(Cn3ccc(=[N+](C)C)c4ccc(Cl)cc34)c2)c2cc(Cl)ccc12 |(-4.13,5.6,;-2.77,4.89,;-1.46,5.71,;-2.7,3.35,;-1.34,2.64,;-1.27,1.09,;-2.58,.27,;-2.52,-1.27,;-1.16,-1.99,;-1.97,-3.29,;-1.24,-4.64,;.3,-4.67,;1.1,-3.36,;.36,-2.01,;2.64,-3.38,;3.38,-4.73,;4.92,-4.77,;5.72,-3.44,;4.97,-2.1,;6.28,-1.29,;6.23,.25,;4.88,.98,;4.86,2.53,;6.16,3.33,;6.13,4.87,;7.45,5.68,;4.79,5.61,;7.52,2.59,;8.82,3.38,;10.18,2.65,;10.22,1.11,;11.57,.37,;8.91,.3,;7.56,1.04,;3.44,-2.06,;-3.94,.99,;-5.25,.16,;-6.62,.89,;-7.92,.07,;-6.66,2.43,;-5.36,3.24,;-4,2.53,)| Show InChI InChI=1S/C36H34Cl2N4/c1-39(2)33-15-17-41(35-21-29(37)11-13-31(33)35)23-25-7-5-9-27(19-25)28-10-6-8-26(20-28)24-42-18-16-34(40(3)4)32-14-12-30(38)22-36(32)42/h5-22H,23-24H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166170
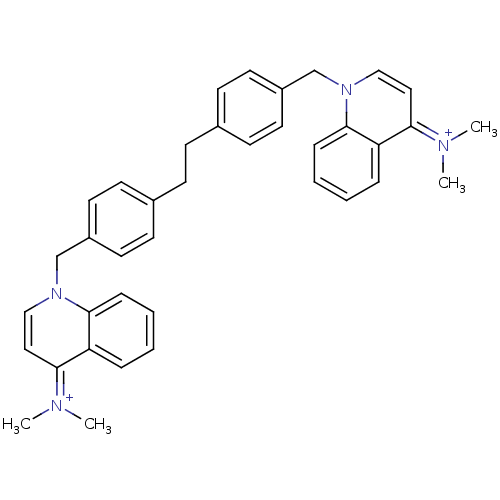
(Bisquinolinium derivative | CHEMBL192987)Show SMILES C[N+](C)=c1ccn(Cc2ccc(CCc3ccc(Cn4ccc(=[N+](C)C)c5ccccc45)cc3)cc2)c2ccccc12 |(-2.72,3.79,;-3.96,2.86,;-5.37,3.45,;-3.76,1.32,;-2.35,.72,;-2.16,-.82,;-3.4,-1.73,;-3.21,-3.27,;-1.8,-3.87,;-1,-2.57,;.54,-2.6,;1.28,-3.94,;2.82,-3.97,;3.57,-5.32,;5.11,-5.35,;5.84,-6.7,;7.38,-6.73,;8.17,-5.41,;9.49,-4.62,;9.46,-3.08,;8.1,-2.34,;8.07,-.78,;9.39,.02,;9.34,1.56,;10.66,2.35,;7.99,2.29,;10.73,-.73,;12.05,.06,;13.4,-.66,;13.44,-2.22,;12.12,-3.02,;10.76,-2.27,;7.43,-4.06,;5.89,-4.04,;.47,-5.26,;-1.07,-5.23,;-4.81,-1.13,;-6.04,-2.06,;-7.46,-1.45,;-7.64,.09,;-6.41,1,;-4.98,.41,)| Show InChI InChI=1S/C38H40N4/c1-39(2)35-23-25-41(37-11-7-5-9-33(35)37)27-31-19-15-29(16-20-31)13-14-30-17-21-32(22-18-30)28-42-26-24-36(40(3)4)34-10-6-8-12-38(34)42/h5-12,15-26H,13-14,27-28H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166192
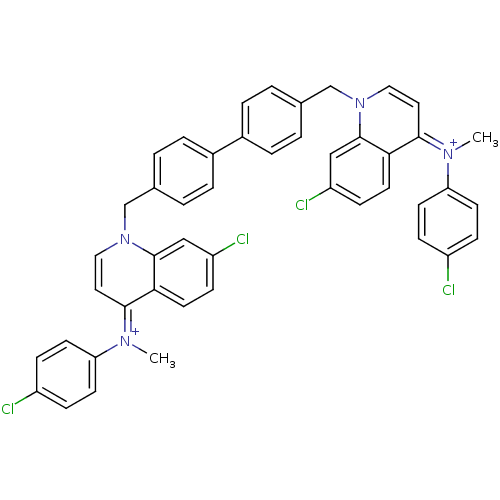
(Bisquinolinium derivative | CHEMBL192063)Show SMILES C\[N+](c1ccc(Cl)cc1)=c1\ccn(Cc2ccc(cc2)-c2ccc(Cn3cc\c(=[N+](/C)c4ccc(Cl)cc4)c4ccc(Cl)cc34)cc2)c2cc(Cl)ccc12 Show InChI InChI=1S/C46H36Cl4N4/c1-51(39-17-11-35(47)12-18-39)43-23-25-53(45-27-37(49)15-21-41(43)45)29-31-3-7-33(8-4-31)34-9-5-32(6-10-34)30-54-26-24-44(42-22-16-38(50)28-46(42)54)52(2)40-19-13-36(48)14-20-40/h3-28H,29-30H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166186

(Bisquinolinium derivative | CHEMBL192091)Show SMILES C\[N+](c1ccccc1)=c1\ccn(Cc2ccc(cc2)-c2ccc(Cn3cc\c(=[N+](/C)c4ccccc4)c4ccc(Cl)cc34)cc2)c2cc(Cl)ccc12 Show InChI InChI=1S/C46H38Cl2N4/c1-49(39-9-5-3-6-10-39)43-25-27-51(45-29-37(47)21-23-41(43)45)31-33-13-17-35(18-14-33)36-19-15-34(16-20-36)32-52-28-26-44(42-24-22-38(48)30-46(42)52)50(2)40-11-7-4-8-12-40/h3-30H,31-32H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166206
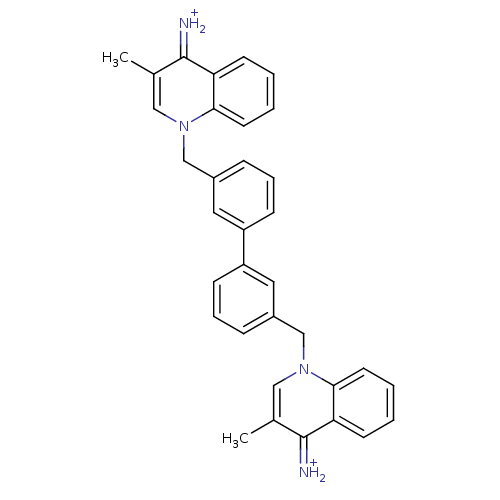
(Bisquinolinium derivative | CHEMBL365345)Show SMILES Cc1cn(Cc2cccc(c2)-c2cccc(Cn3cc(C)c(=[NH2+])c4ccccc34)c2)c2ccccc2c1=[NH2+] Show InChI InChI=1S/C34H30N4/c1-23-19-37(31-15-5-3-13-29(31)33(23)35)21-25-9-7-11-27(17-25)28-12-8-10-26(18-28)22-38-20-24(2)34(36)30-14-4-6-16-32(30)38/h3-20,35-36H,21-22H2,1-2H3/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166182
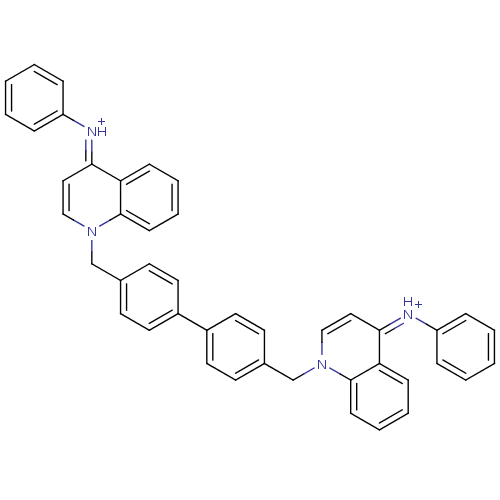
(Bisquinolinium derivative | CHEMBL192874)Show SMILES C(c1ccc(cc1)-c1ccc(Cn2cc\c(=[NH+]/c3ccccc3)c3ccccc23)cc1)n1cc\c(=[NH+]\c2ccccc2)c2ccccc12 Show InChI InChI=1S/C44H34N4/c1-3-11-37(12-4-1)45-41-27-29-47(43-17-9-7-15-39(41)43)31-33-19-23-35(24-20-33)36-25-21-34(22-26-36)32-48-30-28-42(40-16-8-10-18-44(40)48)46-38-13-5-2-6-14-38/h1-30H,31-32H2/p+2/b45-41-,46-42+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166178

(Bisquinolinium derivative | CHEMBL364314)Show SMILES Clc1ccc2c(c1)n(Cc1ccc(cc1)-c1ccc(Cn3ccc(=[N+]4CCCC4)c4ccc(Cl)cc34)cc1)ccc2=[N+]1CCCC1 |(-7.5,-3.8,;-6.2,-2.99,;-6.24,-1.45,;-4.94,-.63,;-3.58,-1.34,;-3.52,-2.88,;-4.83,-3.71,;-2.16,-3.6,;-2.1,-5.14,;-.74,-5.86,;-.02,-7.21,;1.52,-7.25,;2.34,-5.92,;1.59,-4.58,;.07,-4.56,;3.88,-5.95,;4.62,-7.3,;6.14,-7.33,;6.95,-6.02,;8.26,-5.21,;8.23,-3.67,;6.88,-2.94,;6.84,-1.38,;8.16,-.59,;8.12,.95,;9.34,1.88,;8.82,3.34,;7.28,3.3,;6.84,1.82,;9.5,-1.34,;10.82,-.54,;12.16,-1.27,;12.2,-2.81,;13.56,-3.55,;10.9,-3.62,;9.55,-2.88,;6.19,-4.66,;4.66,-4.64,;-.85,-2.78,;-.92,-1.24,;-2.28,-.52,;-2.35,1.02,;-3.63,1.86,;-3.21,3.35,;-1.67,3.41,;-1.14,1.97,)| Show InChI InChI=1S/C40H38Cl2N4/c41-33-13-15-35-37(43-19-1-2-20-43)17-23-45(39(35)25-33)27-29-5-9-31(10-6-29)32-11-7-30(8-12-32)28-46-24-18-38(44-21-3-4-22-44)36-16-14-34(42)26-40(36)46/h5-18,23-26H,1-4,19-22,27-28H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166190
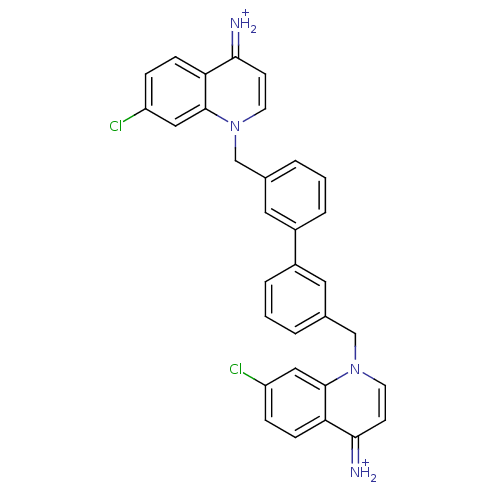
(Bisquinolinium derivative | CHEMBL191818)Show SMILES Clc1ccc2c(c1)n(Cc1cccc(c1)-c1cccc(Cn3ccc(=[NH2+])c4ccc(Cl)cc34)c1)ccc2=[NH2+] Show InChI InChI=1S/C32H24Cl2N4/c33-25-7-9-27-29(35)11-13-37(31(27)17-25)19-21-3-1-5-23(15-21)24-6-2-4-22(16-24)20-38-14-12-30(36)28-10-8-26(34)18-32(28)38/h1-18,35-36H,19-20H2/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166200
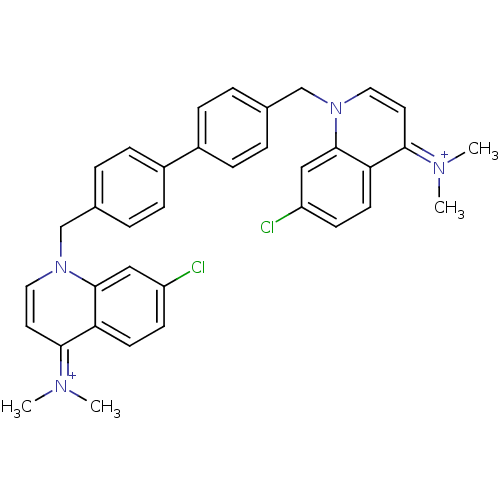
(Bisquinolinium derivative | CHEMBL363612)Show SMILES C[N+](C)=c1ccn(Cc2ccc(cc2)-c2ccc(Cn3ccc(=[N+](C)C)c4ccc(Cl)cc34)cc2)c2cc(Cl)ccc12 |(-1.04,1.83,;-2.35,1.02,;-3.71,1.73,;-2.28,-.52,;-.93,-1.24,;-.86,-2.78,;-2.16,-3.6,;-2.1,-5.14,;-.74,-5.86,;-.02,-7.21,;1.52,-7.25,;2.34,-5.92,;1.59,-4.58,;.07,-4.56,;3.88,-5.95,;4.62,-7.3,;6.14,-7.33,;6.95,-6.02,;8.26,-5.21,;8.23,-3.67,;6.88,-2.94,;6.84,-1.38,;8.16,-.59,;8.12,.95,;9.43,1.75,;6.77,1.68,;9.5,-1.34,;10.82,-.54,;12.16,-1.27,;12.2,-2.81,;13.56,-3.55,;10.9,-3.62,;9.55,-2.88,;6.19,-4.66,;4.66,-4.64,;-3.52,-2.88,;-4.83,-3.71,;-6.2,-2.99,;-7.5,-3.8,;-6.24,-1.45,;-4.94,-.63,;-3.58,-1.34,)| Show InChI InChI=1S/C36H34Cl2N4/c1-39(2)33-17-19-41(35-21-29(37)13-15-31(33)35)23-25-5-9-27(10-6-25)28-11-7-26(8-12-28)24-42-20-18-34(40(3)4)32-16-14-30(38)22-36(32)42/h5-22H,23-24H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166187
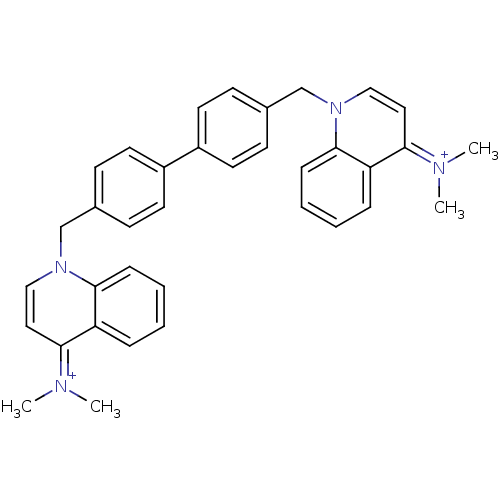
(Bisquinolinium derivative | CHEMBL192105)Show SMILES C[N+](C)=c1ccn(Cc2ccc(cc2)-c2ccc(Cn3ccc(=[N+](C)C)c4ccccc34)cc2)c2ccccc12 |(-5.3,3.61,;-3.89,3,;-2.65,3.92,;-3.69,1.46,;-2.28,.86,;-2.09,-.68,;-3.33,-1.59,;-3.14,-3.13,;-1.73,-3.73,;-1,-5.09,;.54,-5.12,;1.35,-3.8,;.61,-2.46,;-.93,-2.43,;2.88,-3.83,;3.67,-2.52,;5.21,-2.54,;5.96,-3.9,;7.28,-3.1,;7.24,-1.56,;5.89,-.82,;5.86,.74,;7.17,1.53,;7.12,3.07,;8.45,3.86,;5.79,3.82,;8.51,.79,;9.83,1.58,;11.18,.86,;11.22,-.7,;9.9,-1.5,;8.55,-.75,;5.16,-5.21,;3.62,-5.19,;-4.74,-.99,;-5.97,-1.92,;-7.39,-1.31,;-7.56,.23,;-6.34,1.14,;-4.91,.55,)| Show InChI InChI=1S/C36H36N4/c1-37(2)33-21-23-39(35-11-7-5-9-31(33)35)25-27-13-17-29(18-14-27)30-19-15-28(16-20-30)26-40-24-22-34(38(3)4)32-10-6-8-12-36(32)40/h5-24H,25-26H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166207

(Bisquinolinium derivative | CHEMBL370702)Show SMILES Cc1c(N)ccc2c1n(Cc1ccc(cc1)-c1ccc(Cn3cc\c(=[N+](/C)c4ccc(Cl)cc4)c4ccc(N)c(C)c34)cc1)cc\c2=[N+](\C)c1ccc(Cl)cc1 Show InChI InChI=1S/C48H42Cl2N6/c1-31-43(51)23-21-41-45(53(3)39-17-13-37(49)14-18-39)25-27-55(47(31)41)29-33-5-9-35(10-6-33)36-11-7-34(8-12-36)30-56-28-26-46(42-22-24-44(52)32(2)48(42)56)54(4)40-19-15-38(50)16-20-40/h5-28,51-52H,29-30H2,1-4H3/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166201
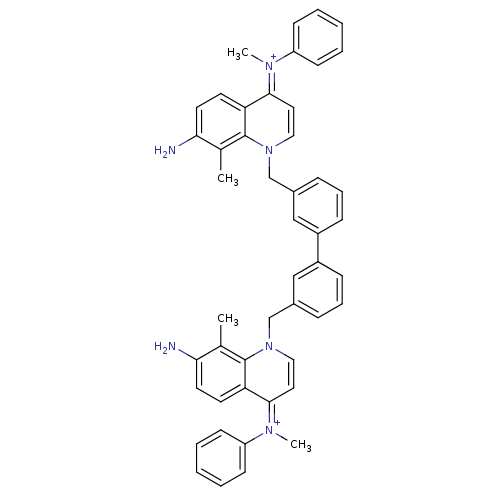
(Bisquinolinium derivative | CHEMBL192379)Show SMILES Cc1c(N)ccc2c1n(Cc1cccc(c1)-c1cccc(Cn3cc\c(=[N+](/C)c4ccccc4)c4ccc(N)c(C)c34)c1)cc\c2=[N+](\C)c1ccccc1 Show InChI InChI=1S/C48H44N6/c1-33-43(49)23-21-41-45(51(3)39-17-7-5-8-18-39)25-27-53(47(33)41)31-35-13-11-15-37(29-35)38-16-12-14-36(30-38)32-54-28-26-46(52(4)40-19-9-6-10-20-40)42-22-24-44(50)34(2)48(42)54/h5-30,49-50H,31-32H2,1-4H3/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166179
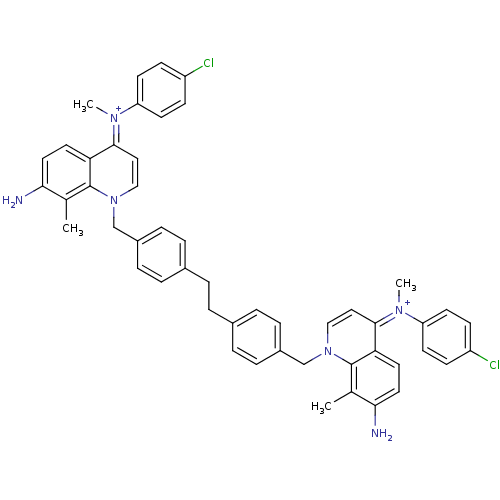
(Bisquinolinium derivative | CHEMBL372647)Show SMILES Cc1c(N)ccc2c1n(Cc1ccc(CCc3ccc(Cn4cc\c(=[N+](/C)c5ccc(Cl)cc5)c5ccc(N)c(C)c45)cc3)cc1)cc\c2=[N+](\C)c1ccc(Cl)cc1 Show InChI InChI=1S/C50H46Cl2N6/c1-33-45(53)25-23-43-47(55(3)41-19-15-39(51)16-20-41)27-29-57(49(33)43)31-37-11-7-35(8-12-37)5-6-36-9-13-38(14-10-36)32-58-30-28-48(44-24-26-46(54)34(2)50(44)58)56(4)42-21-17-40(52)18-22-42/h7-30,53-54H,5-6,31-32H2,1-4H3/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166191

(Bisquinolinium derivative | CHEMBL192631)Show SMILES Clc1ccc2c(c1)n(Cc1ccc(cc1)-c1ccc(Cn3ccc(=[NH2+])c4ccc(Cl)cc34)cc1)ccc2=[NH2+] Show InChI InChI=1S/C32H24Cl2N4/c33-25-9-11-27-29(35)13-15-37(31(27)17-25)19-21-1-5-23(6-2-21)24-7-3-22(4-8-24)20-38-16-14-30(36)28-12-10-26(34)18-32(28)38/h1-18,35-36H,19-20H2/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50054095
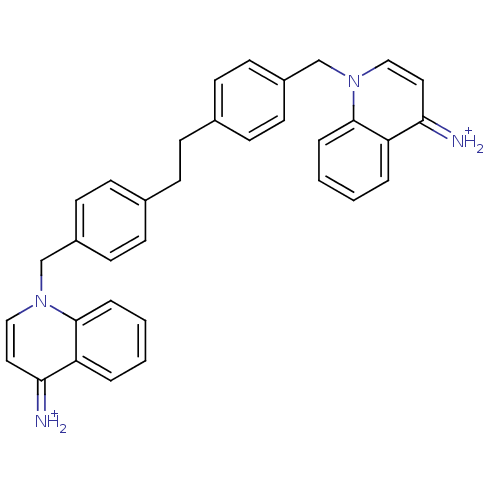
(1-{4-[4-(4-amino-1-quinoliniumylmethyl)phenethyl]b...)Show SMILES [NH2+]=c1ccn(Cc2ccc(CCc3ccc(Cn4ccc(=[NH2+])c5ccccc45)cc3)cc2)c2ccccc12 Show InChI InChI=1S/C34H30N4/c35-31-19-21-37(33-7-3-1-5-29(31)33)23-27-15-11-25(12-16-27)9-10-26-13-17-28(18-14-26)24-38-22-20-32(36)30-6-2-4-8-34(30)38/h1-8,11-22,35-36H,9-10,23-24H2/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50054103

(1,1'-[biphenyl-4,4'-diylbis(methylene)]-bis(4-amin...)Show SMILES [NH2+]=c1ccn(Cc2ccc(cc2)-c2ccc(Cn3ccc(=[NH2+])c4ccccc34)cc2)c2ccccc12 Show InChI InChI=1S/C32H26N4/c33-29-17-19-35(31-7-3-1-5-27(29)31)21-23-9-13-25(14-10-23)26-15-11-24(12-16-26)22-36-20-18-30(34)28-6-2-4-8-32(28)36/h1-20,33-34H,21-22H2/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166203
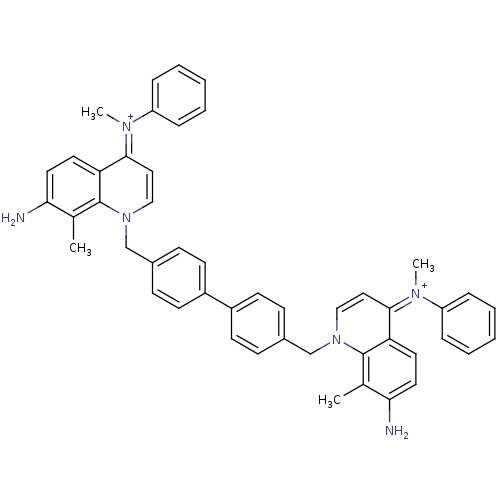
(Bisquinolinium derivative | CHEMBL372679)Show SMILES Cc1c(N)ccc2c1n(Cc1ccc(cc1)-c1ccc(Cn3cc\c(=[N+](/C)c4ccccc4)c4ccc(N)c(C)c34)cc1)cc\c2=[N+](\C)c1ccccc1 Show InChI InChI=1S/C48H44N6/c1-33-43(49)25-23-41-45(51(3)39-11-7-5-8-12-39)27-29-53(47(33)41)31-35-15-19-37(20-16-35)38-21-17-36(18-22-38)32-54-30-28-46(52(4)40-13-9-6-10-14-40)42-24-26-44(50)34(2)48(42)54/h5-30,49-50H,31-32H2,1-4H3/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166173
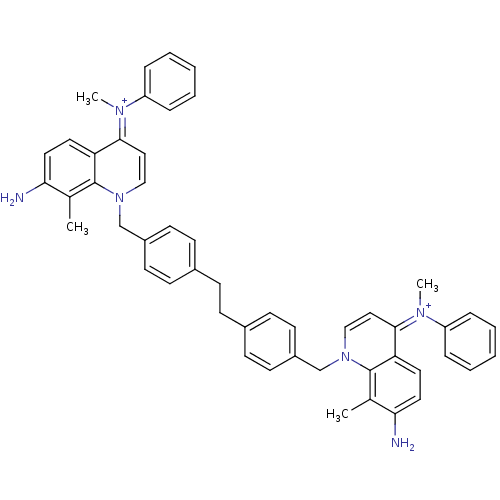
(Bisquinolinium derivative | CHEMBL193262)Show SMILES Cc1c(N)ccc2c1n(Cc1ccc(CCc3ccc(Cn4cc\c(=[N+](/C)c5ccccc5)c5ccc(N)c(C)c45)cc3)cc1)cc\c2=[N+](\C)c1ccccc1 Show InChI InChI=1S/C50H48N6/c1-35-45(51)27-25-43-47(53(3)41-11-7-5-8-12-41)29-31-55(49(35)43)33-39-21-17-37(18-22-39)15-16-38-19-23-40(24-20-38)34-56-32-30-48(54(4)42-13-9-6-10-14-42)44-26-28-46(52)36(2)50(44)56/h5-14,17-32,51-52H,15-16,33-34H2,1-4H3/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166197
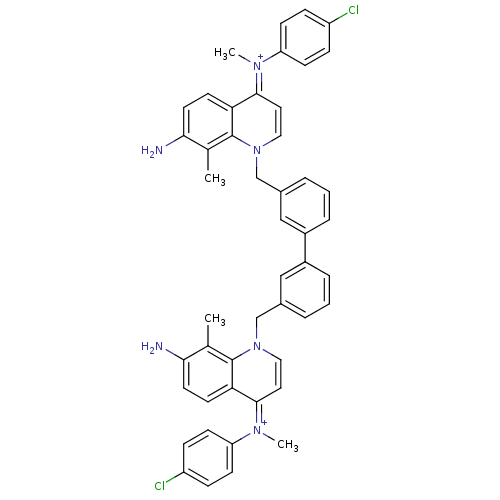
(Bisquinolinium derivative | CHEMBL426637)Show SMILES Cc1c(N)ccc2c1n(Cc1cccc(c1)-c1cccc(Cn3cc\c(=[N+](/C)c4ccc(Cl)cc4)c4ccc(N)c(C)c34)c1)cc\c2=[N+](\C)c1ccc(Cl)cc1 Show InChI InChI=1S/C48H42Cl2N6/c1-31-43(51)21-19-41-45(53(3)39-15-11-37(49)12-16-39)23-25-55(47(31)41)29-33-7-5-9-35(27-33)36-10-6-8-34(28-36)30-56-26-24-46(42-20-22-44(52)32(2)48(42)56)54(4)40-17-13-38(50)14-18-40/h5-28,51-52H,29-30H2,1-4H3/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166209
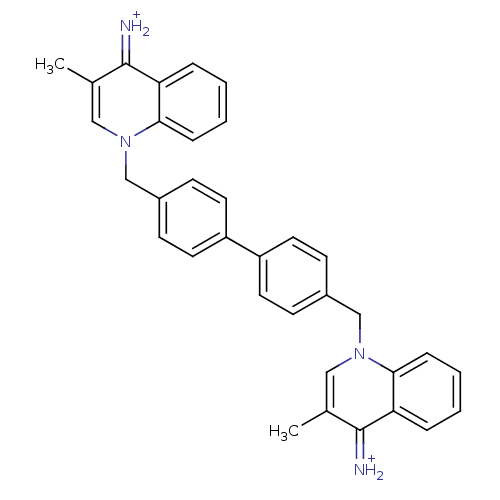
(Bisquinolinium derivative | CHEMBL364075)Show SMILES Cc1cn(Cc2ccc(cc2)-c2ccc(Cn3cc(C)c(=[NH2+])c4ccccc34)cc2)c2ccccc2c1=[NH2+] Show InChI InChI=1S/C34H30N4/c1-23-19-37(31-9-5-3-7-29(31)33(23)35)21-25-11-15-27(16-12-25)28-17-13-26(14-18-28)22-38-20-24(2)34(36)30-8-4-6-10-32(30)38/h3-20,35-36H,21-22H2,1-2H3/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50166196

(Bisquinolinium derivative | CHEMBL371151)Show SMILES Clc1ccc2c(c1)n(Cc1ccc(CCc3ccc(Cn4ccc(=[NH2+])c5ccc(Cl)cc45)cc3)cc1)ccc2=[NH2+] Show InChI InChI=1S/C34H28Cl2N4/c35-27-11-13-29-31(37)15-17-39(33(29)19-27)21-25-7-3-23(4-8-25)1-2-24-5-9-26(10-6-24)22-40-18-16-32(38)30-14-12-28(36)20-34(30)40/h3-20,37-38H,1-2,21-22H2/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Ex vivo inhibitory concentration against human choline kinase |
J Med Chem 48: 3354-63 (2005)
Article DOI: 10.1021/jm049061o
BindingDB Entry DOI: 10.7270/Q2WD403P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data