 Found 6 hits Enz. Inhib. hit(s) with all data for entry = 50016418
Found 6 hits Enz. Inhib. hit(s) with all data for entry = 50016418 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114068
BindingDB Entry DOI: 10.7270/Q29W0KH4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50594814
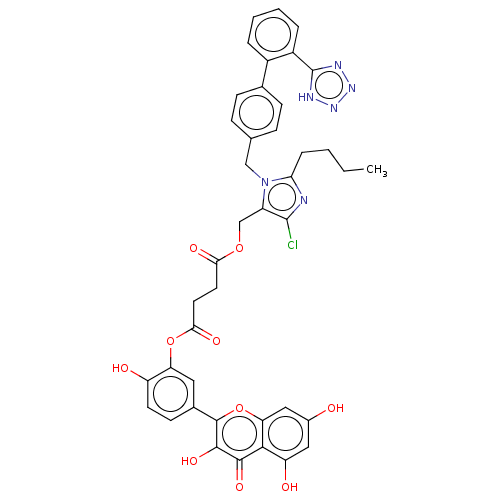
(CHEMBL5192190)Show SMILES CCCCc1nc(Cl)c(COC(=O)CCC(=O)Oc2cc(ccc2O)-c2oc3cc(O)cc(O)c3c(=O)c2O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114068
BindingDB Entry DOI: 10.7270/Q29W0KH4 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50594815
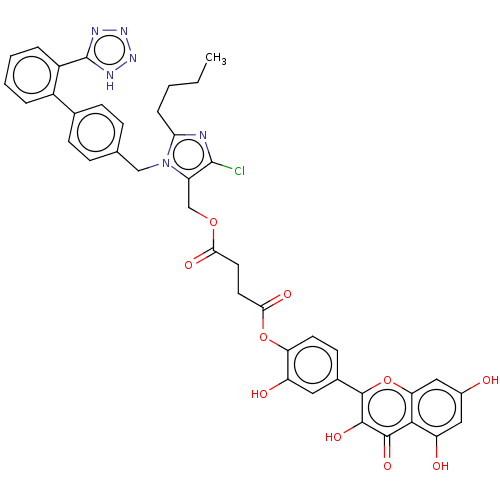
(CHEMBL5203983)Show SMILES CCCCc1nc(Cl)c(COC(=O)CCC(=O)Oc2ccc(cc2O)-c2oc3cc(O)cc(O)c3c(=O)c2O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114068
BindingDB Entry DOI: 10.7270/Q29W0KH4 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114068
BindingDB Entry DOI: 10.7270/Q29W0KH4 |
More data for this
Ligand-Target Pair | |
UDP-galactopyranose mutase
(Mycobacterium tuberculosis H37Rv) | BDBM50594816
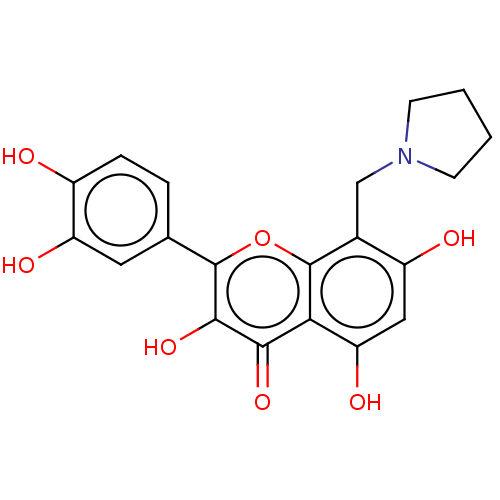
(CHEMBL5205613)Show SMILES Cl.Oc1ccc(cc1O)-c1oc2c(CN3CCCC3)c(O)cc(O)c2c(=O)c1O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114068
BindingDB Entry DOI: 10.7270/Q29W0KH4 |
More data for this
Ligand-Target Pair | |
UDP-galactopyranose mutase
(Mycobacterium tuberculosis H37Rv) | BDBM50262559
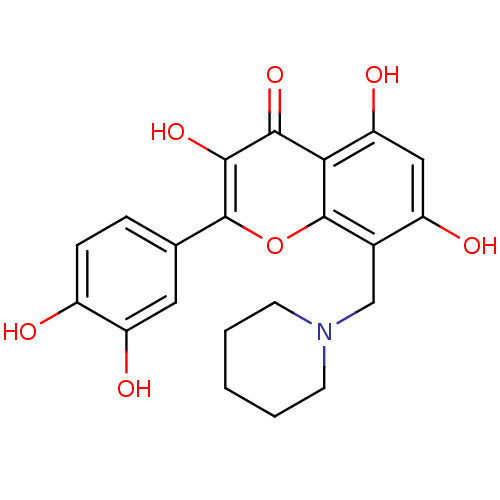
(2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-8-(piperi...)Show SMILES Oc1ccc(cc1O)-c1oc2c(CN3CCCCC3)c(O)cc(O)c2c(=O)c1O Show InChI InChI=1S/C21H21NO7/c23-13-5-4-11(8-15(13)25)20-19(28)18(27)17-16(26)9-14(24)12(21(17)29-20)10-22-6-2-1-3-7-22/h4-5,8-9,23-26,28H,1-3,6-7,10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114068
BindingDB Entry DOI: 10.7270/Q29W0KH4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data