 Found 73 hits Enz. Inhib. hit(s) with all data for entry = 50018240
Found 73 hits Enz. Inhib. hit(s) with all data for entry = 50018240 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50188594
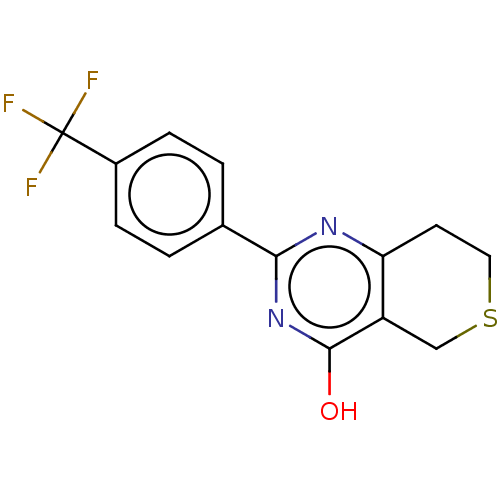
(CHEBI:62878 | CHEMBL1086580)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-5-6-21-7-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50188594

(CHEBI:62878 | CHEMBL1086580)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-5-6-21-7-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Catenin beta-1
(Homo sapiens (Human)) | BDBM50094490
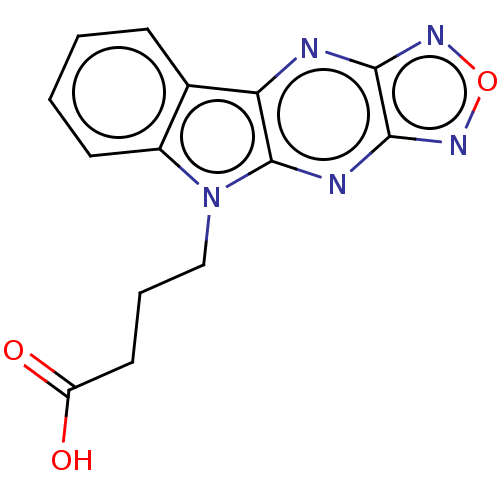
(CHEMBL3589156)Show InChI InChI=1S/C14H11N5O3/c20-10(21)6-3-7-19-9-5-2-1-4-8(9)11-14(19)16-13-12(15-11)17-22-18-13/h1-2,4-5H,3,6-7H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Catenin beta-1
(Homo sapiens (Human)) | BDBM50094590

(CHEMBL3589012)Show SMILES Nc1nonc1C(=O)N\N=C1\c2ccccc2-c2nc3nonc3nc12 Show InChI InChI=1S/C22H18N4S/c1-14(15-8-3-2-4-9-15)26-17-11-6-5-10-16(17)19-20(23)24-21(25-22(19)26)18-12-7-13-27-18/h2-14H,1H3,(H2,23,24,25)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Catenin beta-1
(Homo sapiens (Human)) | BDBM50094510
(CHEMBL3589019)Show InChI InChI=1S/C14H9N9O3/c15-10-11(20-25-19-10)16-8(24)5-23-7-4-2-1-3-6(7)9-14(23)18-13-12(17-9)21-26-22-13/h1-4H,5H2,(H2,15,19)(H,16,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50408056
(CHEMBL5274911)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1cccs1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@H](-[#6]-1-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O)-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C55H91N19O13S/c56-35(16-6-20-63-53(57)58)45(79)69-36(17-7-21-64-54(59)60)49(83)72-23-9-19-40(72)50(84)74-28-33(76)25-41(74)48(82)66-27-42(77)68-38(26-34-15-10-24-88-34)46(80)70-39(30-75)47(81)71-44(31-11-4-5-12-31)51(85)73(32-13-2-1-3-14-32)29-43(78)67-37(52(86)87)18-8-22-65-55(61)62/h10,15,24,31-33,35-41,44,75-76H,1-9,11-14,16-23,25-30,56H2,(H,66,82)(H,67,78)(H,68,77)(H,69,79)(H,70,80)(H,71,81)(H,86,87)(H4,57,58,63)(H4,59,60,64)(H4,61,62,65)/t33-,35+,36+,37+,38+,39+,40+,41+,44-/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against bradykinin B2 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Catenin beta-1
(Homo sapiens (Human)) | BDBM50094486
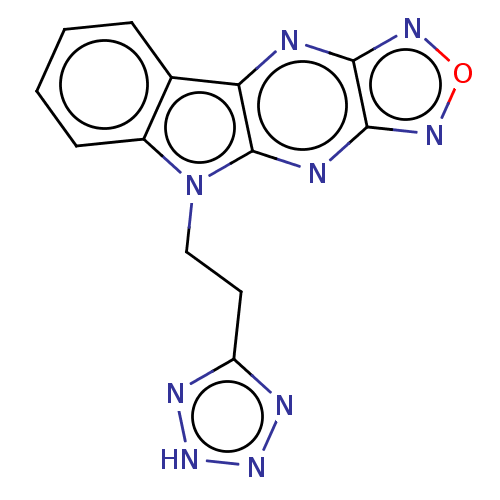
(CHEMBL3589160)Show InChI InChI=1S/C13H9N9O/c1-2-4-8-7(3-1)10-13(15-12-11(14-10)18-23-19-12)22(8)6-5-9-16-20-21-17-9/h1-4H,5-6H2,(H,16,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50407813

(CHEMBL5194149)Show SMILES [#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C37H50N8O7/c38-26(17-22-9-2-1-3-10-22)32(47)43-28(21-46)34(49)44-20-25-13-5-4-11-23(25)18-31(44)35(50)45-29-15-7-6-12-24(29)19-30(45)33(48)42-27(36(51)52)14-8-16-41-37(39)40/h1-5,9-11,13,24,26-31,46H,6-8,12,14-21,38H2,(H,42,48)(H,43,47)(H,51,52)(H4,39,40,41)/t24?,26-,27-,28-,29?,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at cloned recombinant human adenosine A2B receptor transfected in CHO cells by cAMP assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50234915
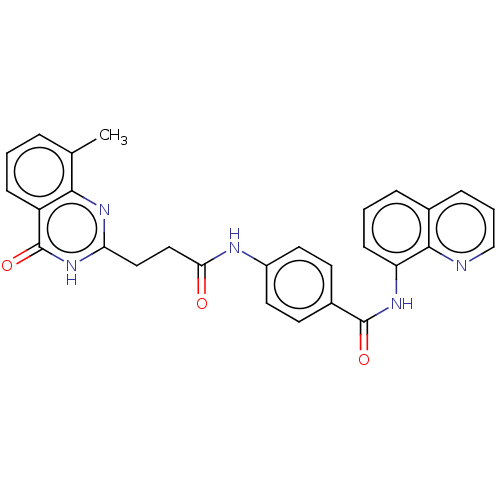
(CHEMBL4069447)Show SMILES Cc1cccc2c1nc(CCC(=O)Nc1ccc(cc1)C(=O)Nc1cccc3cccnc13)[nH]c2=O Show InChI InChI=1S/C28H23N5O3/c1-17-5-2-8-21-25(17)32-23(33-28(21)36)14-15-24(34)30-20-12-10-19(11-13-20)27(35)31-22-9-3-6-18-7-4-16-29-26(18)22/h2-13,16H,14-15H2,1H3,(H,30,34)(H,31,35)(H,32,33,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50234916
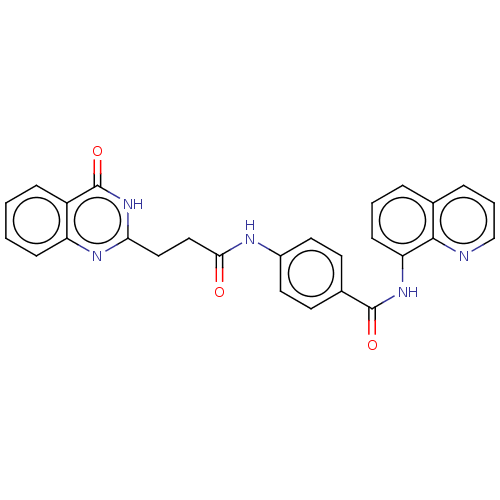
(CHEMBL4098188)Show SMILES O=C(CCc1nc2ccccc2c(=O)[nH]1)Nc1ccc(cc1)C(=O)Nc1cccc2cccnc12 Show InChI InChI=1S/C27H21N5O3/c33-24(15-14-23-30-21-8-2-1-7-20(21)27(35)32-23)29-19-12-10-18(11-13-19)26(34)31-22-9-3-5-17-6-4-16-28-25(17)22/h1-13,16H,14-15H2,(H,29,33)(H,31,34)(H,30,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stefin-3
(Mus musculus) | BDBM50233628
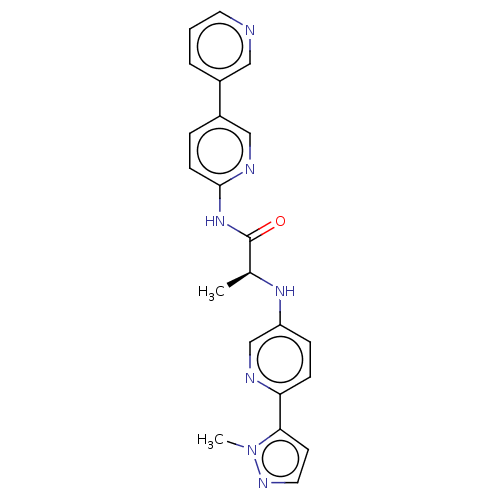
(CHEMBL4088716)Show SMILES C[C@H](Nc1ccc(nc1)-c1ccnn1C)C(=O)Nc1ccc(cn1)-c1cccnc1 |r| Show InChI InChI=1S/C22H21N7O/c1-15(27-18-6-7-19(24-14-18)20-9-11-26-29(20)2)22(30)28-21-8-5-17(13-25-21)16-4-3-10-23-12-16/h3-15,27H,1-2H3,(H,25,28,30)/t15-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Stefin-3
(Mus musculus) | BDBM50233639

(CHEMBL4097104)Show SMILES O=C(COc1ccc(cc1)-n1ccnc1)Nc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C23H19N3O2/c27-23(16-28-22-12-10-21(11-13-22)26-15-14-24-17-26)25-20-8-6-19(7-9-20)18-4-2-1-3-5-18/h1-15,17H,16H2,(H,25,27) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50444583
(CHEMBL3099716 | US9340549, 74)Show SMILES CC1(C)OC(=O)N([C@H]1c1ccccc1)[C@H]1CC[C@@H](CC1)c1cnc(N)c(c1)-c1ncccn1 |r,wU:17.22,7.8,wD:14.15,(49.17,-3.5,;50.51,-4.26,;49.19,-5.04,;51.4,-3,;52.87,-3.46,;54.11,-2.55,;52.89,-5,;51.43,-5.5,;50.7,-6.85,;49.16,-6.89,;48.43,-8.25,;49.24,-9.56,;50.79,-9.51,;51.51,-8.15,;54.14,-5.9,;54.14,-7.45,;55.47,-8.22,;56.81,-7.45,;56.8,-5.9,;55.47,-5.13,;58.14,-8.21,;59.46,-7.44,;60.8,-8.2,;60.8,-9.75,;62.14,-10.52,;59.47,-10.52,;58.14,-9.76,;59.47,-12.05,;58.14,-12.83,;58.14,-14.36,;59.47,-15.13,;60.81,-14.35,;60.8,-12.82,)| Show InChI InChI=1S/C26H29N5O2/c1-26(2)22(18-7-4-3-5-8-18)31(25(32)33-26)20-11-9-17(10-12-20)19-15-21(23(27)30-16-19)24-28-13-6-14-29-24/h3-8,13-17,20,22H,9-12H2,1-2H3,(H2,27,30)/t17-,20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50425969
(CHEMBL2314698)Show SMILES COc1ccccc1NC(=O)c1ccc(NC(=O)CCc2nc3ccccc3c(=O)[nH]2)cc1 Show InChI InChI=1S/C25H22N4O4/c1-33-21-9-5-4-8-20(21)28-24(31)16-10-12-17(13-11-16)26-23(30)15-14-22-27-19-7-3-2-6-18(19)25(32)29-22/h2-13H,14-15H2,1H3,(H,26,30)(H,28,31)(H,27,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50188594

(CHEBI:62878 | CHEMBL1086580)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-5-6-21-7-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50188594

(CHEBI:62878 | CHEMBL1086580)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-5-6-21-7-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Catenin beta-1
(Homo sapiens (Human)) | BDBM50408050
(CHEMBL5282944)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1cccs1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O)-c1ccccc1 Show InChI InChI=1S/C57H83N19O13S/c58-37(17-7-21-65-55(59)60)47(81)71-38(18-8-22-66-56(61)62)51(85)74-24-10-20-43(74)53(87)76-30-35(78)27-44(76)50(84)68-29-45(79)70-40(28-36-16-11-25-90-36)48(82)73-42(32-77)49(83)72-41(26-33-12-3-1-4-13-33)52(86)75(34-14-5-2-6-15-34)31-46(80)69-39(54(88)89)19-9-23-67-57(63)64/h1-6,11-16,25,35,37-44,77-78H,7-10,17-24,26-32,58H2,(H,68,84)(H,69,80)(H,70,79)(H,71,81)(H,72,83)(H,73,82)(H,88,89)(H4,59,60,65)(H4,61,62,66)(H4,63,64,67)/t35-,37+,38+,39+,40+,41-,42+,43+,44+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50407965
(CHEMBL3763870)Show SMILES Clc1ccc2Oc3ccccc3CN(C(=O)CNC(=O)CCSCc3ccco3)c2c1 Show InChI InChI=1S/C23H21ClN2O4S/c24-17-7-8-21-19(12-17)26(14-16-4-1-2-6-20(16)30-21)23(28)13-25-22(27)9-11-31-15-18-5-3-10-29-18/h1-8,10,12H,9,11,13-15H2,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50444543
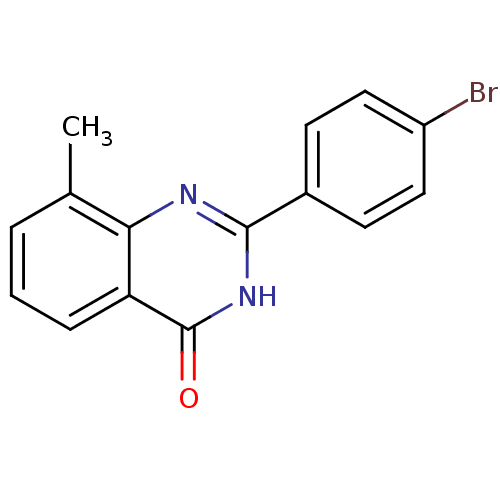
(CHEMBL3098937)Show InChI InChI=1S/C15H11BrN2O/c1-9-3-2-4-12-13(9)17-14(18-15(12)19)10-5-7-11(16)8-6-10/h2-8H,1H3,(H,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50094941
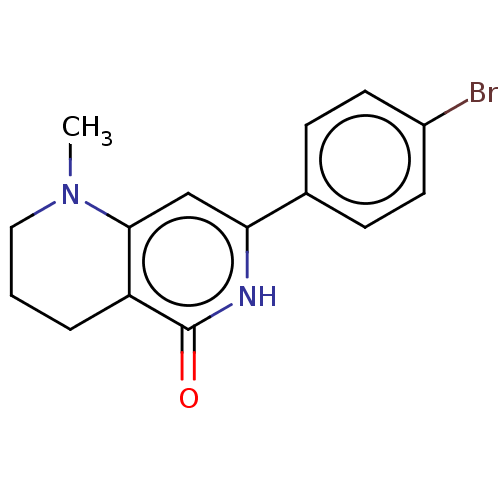
(CHEMBL3589285)Show InChI InChI=1S/C18H15N3O/c1-20-18(22)15-12-21(11-13-7-3-2-4-8-13)16-10-6-5-9-14(16)17(15)19-20/h2-10,12H,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50505333
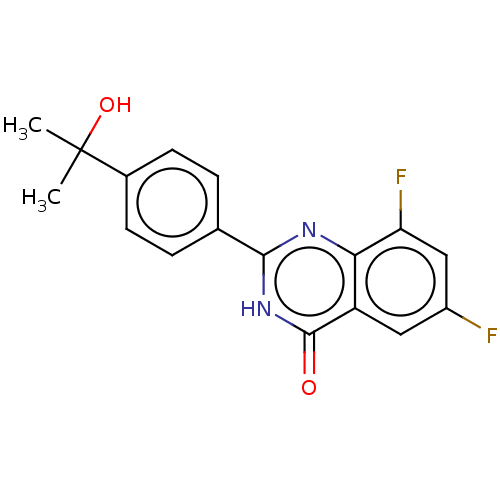
(CHEMBL4437173)Show SMILES CC(C)(O)c1ccc(cc1)-c1nc2c(F)cc(F)cc2c(=O)[nH]1 Show InChI InChI=1S/C17H14F2N2O2/c1-17(2,23)10-5-3-9(4-6-10)15-20-14-12(16(22)21-15)7-11(18)8-13(14)19/h3-8,23H,1-2H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50407966
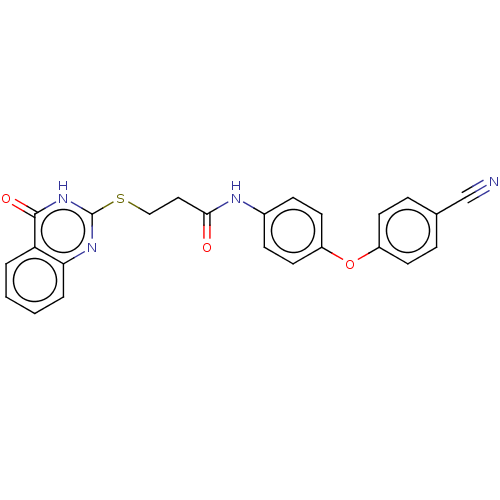
(CHEMBL5274581)Show SMILES CCS(=O)(=O)CCC(=O)NCC(=O)N1Cc2ccccc2Oc2ccc(Cl)cc12 Show InChI InChI=1S/C20H21ClN2O5S/c1-2-29(26,27)10-9-19(24)22-12-20(25)23-13-14-5-3-4-6-17(14)28-18-8-7-15(21)11-16(18)23/h3-8,11H,2,9-10,12-13H2,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50425969

(CHEMBL2314698)Show SMILES COc1ccccc1NC(=O)c1ccc(NC(=O)CCc2nc3ccccc3c(=O)[nH]2)cc1 Show InChI InChI=1S/C25H22N4O4/c1-33-21-9-5-4-8-20(21)28-24(31)16-10-12-17(13-11-16)26-23(30)15-14-22-27-19-7-3-2-6-18(19)25(32)29-22/h2-13H,14-15H2,1H3,(H,26,30)(H,28,31)(H,27,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50407814
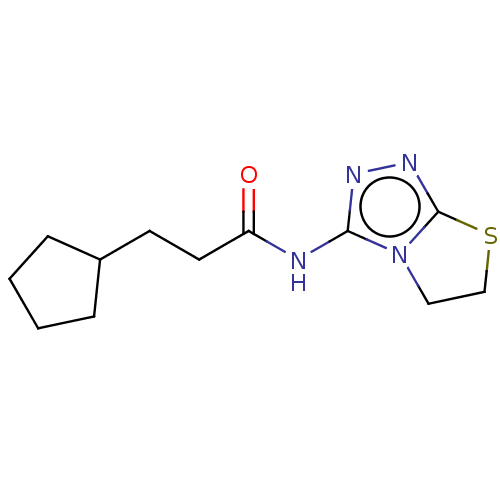
(CHEMBL2425800)Show SMILES CN1CC(=O)N[C@@H](Cc2cccs2)C(=O)N2Cc3ccccc3C[C@@H]2C(=O)N2C3CCCCC3C[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C1=O |r| Show InChI InChI=1S/C35H46N8O5S/c1-41-20-30(44)39-26(18-24-11-7-15-49-24)33(47)42-19-23-10-3-2-8-21(23)16-29(42)34(48)43-27-13-5-4-9-22(27)17-28(43)31(45)40-25(32(41)46)12-6-14-38-35(36)37/h2-3,7-8,10-11,15,22,25-29H,4-6,9,12-14,16-20H2,1H3,(H,39,44)(H,40,45)(H4,36,37,38)/t22?,25-,26-,27?,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50505333

(CHEMBL4437173)Show SMILES CC(C)(O)c1ccc(cc1)-c1nc2c(F)cc(F)cc2c(=O)[nH]1 Show InChI InChI=1S/C17H14F2N2O2/c1-17(2,23)10-5-3-9(4-6-10)15-20-14-12(16(22)21-15)7-11(18)8-13(14)19/h3-8,23H,1-2H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50188594

(CHEBI:62878 | CHEMBL1086580)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-5-6-21-7-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stefin-3
(Mus musculus) | BDBM50257128

(CHEMBL4087906)Show SMILES O=C(CN1CCCC2=C1C(=O)N(C1CC1)C2=O)Nc1ccc(nn1)-c1cccnc1 |c:7| Show InChI InChI=1S/C21H20N6O3/c28-18(23-17-8-7-16(24-25-17)13-3-1-9-22-11-13)12-26-10-2-4-15-19(26)21(30)27(20(15)29)14-5-6-14/h1,3,7-9,11,14H,2,4-6,10,12H2,(H,23,25,28) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Stefin-3
(Mus musculus) | BDBM50257137
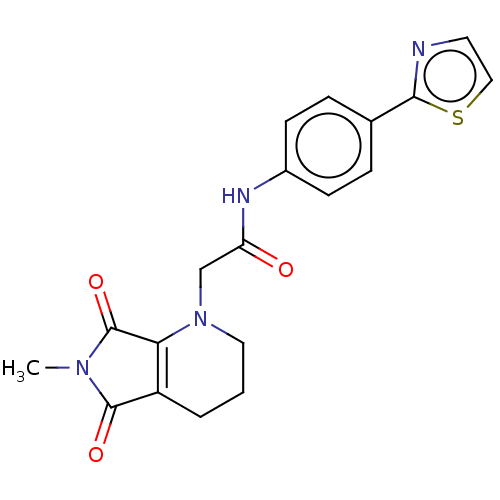
(CHEMBL4094309)Show SMILES CN1C(=O)C2=C(N(CC(=O)Nc3ccc(cc3)-c3nccs3)CCC2)C1=O |t:4| Show InChI InChI=1S/C19H18N4O3S/c1-22-18(25)14-3-2-9-23(16(14)19(22)26)11-15(24)21-13-6-4-12(5-7-13)17-20-8-10-27-17/h4-8,10H,2-3,9,11H2,1H3,(H,21,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50408057
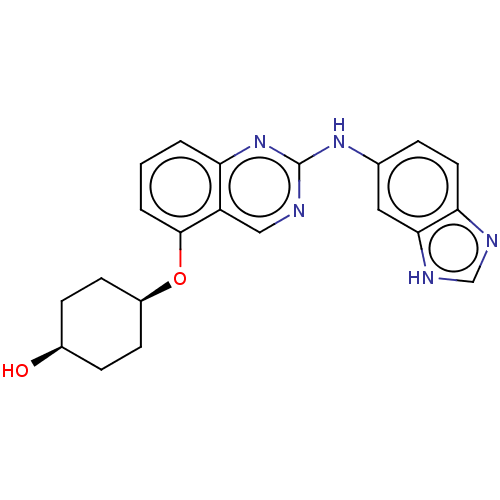
(CHEMBL5272203)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@@H](-[#8])-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@@H]-1-[#6](=O)-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O)-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C60H91N19O13/c61-40(19-9-23-68-58(62)63)50(84)74-41(20-10-24-69-59(64)65)53(87)76-26-12-22-45(76)55(89)79-32-39(81)29-46(79)52(86)71-30-48(82)73-43(27-35-13-3-1-4-14-35)51(85)75-44(34-80)54(88)78-31-37-16-8-7-15-36(37)28-47(78)56(90)77(38-17-5-2-6-18-38)33-49(83)72-42(57(91)92)21-11-25-70-60(66)67/h1,3-4,7-8,13-16,38-47,80-81H,2,5-6,9-12,17-34,61H2,(H,71,86)(H,72,83)(H,73,82)(H,74,84)(H,75,85)(H,91,92)(H4,62,63,68)(H4,64,65,69)(H4,66,67,70)/t39-,40-,41-,42-,43-,44-,45+,46+,47+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist effect against 2-methyl-5-HT activity at 5-HT3 receptor in longitudinal muscle myenteric plexus from guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50444583

(CHEMBL3099716 | US9340549, 74)Show SMILES CC1(C)OC(=O)N([C@H]1c1ccccc1)[C@H]1CC[C@@H](CC1)c1cnc(N)c(c1)-c1ncccn1 |r,wU:17.22,7.8,wD:14.15,(49.17,-3.5,;50.51,-4.26,;49.19,-5.04,;51.4,-3,;52.87,-3.46,;54.11,-2.55,;52.89,-5,;51.43,-5.5,;50.7,-6.85,;49.16,-6.89,;48.43,-8.25,;49.24,-9.56,;50.79,-9.51,;51.51,-8.15,;54.14,-5.9,;54.14,-7.45,;55.47,-8.22,;56.81,-7.45,;56.8,-5.9,;55.47,-5.13,;58.14,-8.21,;59.46,-7.44,;60.8,-8.2,;60.8,-9.75,;62.14,-10.52,;59.47,-10.52,;58.14,-9.76,;59.47,-12.05,;58.14,-12.83,;58.14,-14.36,;59.47,-15.13,;60.81,-14.35,;60.8,-12.82,)| Show InChI InChI=1S/C26H29N5O2/c1-26(2)22(18-7-4-3-5-8-18)31(25(32)33-26)20-11-9-17(10-12-20)19-15-21(23(27)30-16-19)24-28-13-6-14-29-24/h3-8,13-17,20,22H,9-12H2,1-2H3,(H2,27,30)/t17-,20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stefin-3
(Mus musculus) | BDBM50257134

(CHEMBL4066798)Show SMILES Cn1c2ncn(CC(=O)Nc3ccc(cc3)-c3nccs3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C18H16N6O3S/c1-22-15-14(17(26)23(2)18(22)27)24(10-20-15)9-13(25)21-12-5-3-11(4-6-12)16-19-7-8-28-16/h3-8,10H,9H2,1-2H3,(H,21,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50407965

(CHEMBL3763870)Show SMILES Clc1ccc2Oc3ccccc3CN(C(=O)CNC(=O)CCSCc3ccco3)c2c1 Show InChI InChI=1S/C23H21ClN2O4S/c24-17-7-8-21-19(12-17)26(14-16-4-1-2-6-20(16)30-21)23(28)13-25-22(27)9-11-31-15-18-5-3-10-29-18/h1-8,10,12H,9,11,13-15H2,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50468269
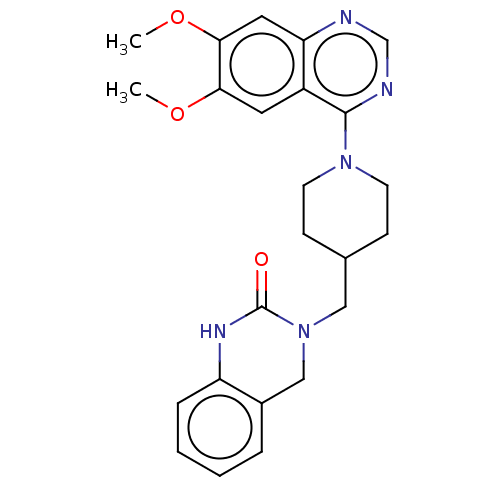
(CHEMBL4285233)Show SMILES COc1cc2ncnc(N3CCC(CN4Cc5ccccc5NC4=O)CC3)c2cc1OC Show InChI InChI=1S/C24H27N5O3/c1-31-21-11-18-20(12-22(21)32-2)25-15-26-23(18)28-9-7-16(8-10-28)13-29-14-17-5-3-4-6-19(17)27-24(29)30/h3-6,11-12,15-16H,7-10,13-14H2,1-2H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50444543

(CHEMBL3098937)Show InChI InChI=1S/C15H11BrN2O/c1-9-3-2-4-12-13(9)17-14(18-15(12)19)10-5-7-11(16)8-6-10/h2-8H,1H3,(H,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50468269

(CHEMBL4285233)Show SMILES COc1cc2ncnc(N3CCC(CN4Cc5ccccc5NC4=O)CC3)c2cc1OC Show InChI InChI=1S/C24H27N5O3/c1-31-21-11-18-20(12-22(21)32-2)25-15-26-23(18)28-9-7-16(8-10-28)13-29-14-17-5-3-4-6-19(17)27-24(29)30/h3-6,11-12,15-16H,7-10,13-14H2,1-2H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sulfotransferase 2A1
(Homo sapiens (Human)) | BDBM50380592
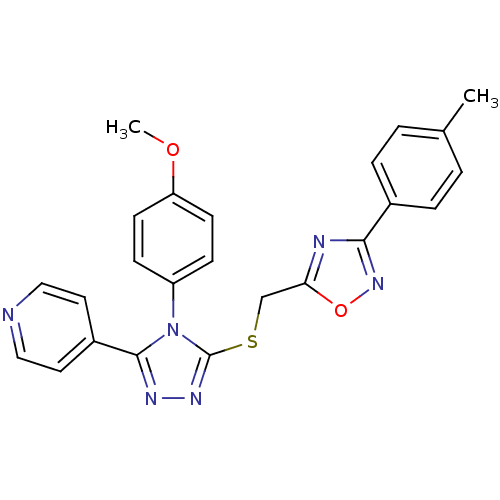
(CHEMBL1552719)Show SMILES COc1ccc(cc1)-n1c(SCc2nc(no2)-c2ccc(C)cc2)nnc1-c1ccncc1 Show InChI InChI=1S/C24H20N6O2S/c1-16-3-5-17(6-4-16)22-26-21(32-29-22)15-33-24-28-27-23(18-11-13-25-14-12-18)30(24)19-7-9-20(31-2)10-8-19/h3-14H,15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50007583
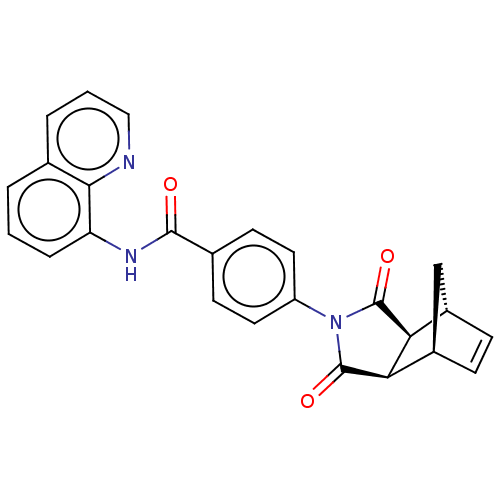
(CHEBI:62882 | CHEMBL562310)Show SMILES [H][C@]12C[C@]([H])(C=C1)[C@]1([H])C(=O)N(C(=O)[C@]21[H])c1ccc(cc1)C(=O)Nc1cccc2cccnc12 |r,c:5| Show InChI InChI=1S/C25H19N3O3/c29-23(27-19-5-1-3-14-4-2-12-26-22(14)19)15-8-10-18(11-9-15)28-24(30)20-16-6-7-17(13-16)21(20)25(28)31/h1-12,16-17,20-21H,13H2,(H,27,29)/t16-,17+,20-,21+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50498905
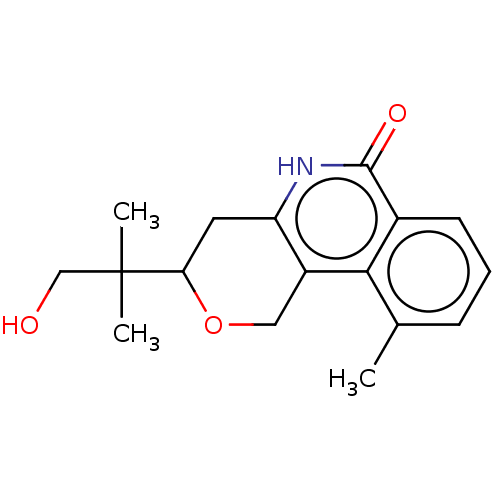
(CHEMBL3736012)Show InChI InChI=1S/C17H21NO3/c1-10-5-4-6-11-15(10)12-8-21-14(17(2,3)9-19)7-13(12)18-16(11)20/h4-6,14,19H,7-9H2,1-3H3,(H,18,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50408057

(CHEMBL5272203)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@@H](-[#8])-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@@H]-1-[#6](=O)-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O)-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C60H91N19O13/c61-40(19-9-23-68-58(62)63)50(84)74-41(20-10-24-69-59(64)65)53(87)76-26-12-22-45(76)55(89)79-32-39(81)29-46(79)52(86)71-30-48(82)73-43(27-35-13-3-1-4-14-35)51(85)75-44(34-80)54(88)78-31-37-16-8-7-15-36(37)28-47(78)56(90)77(38-17-5-2-6-18-38)33-49(83)72-42(57(91)92)21-11-25-70-60(66)67/h1,3-4,7-8,13-16,38-47,80-81H,2,5-6,9-12,17-34,61H2,(H,71,86)(H,72,83)(H,73,82)(H,74,84)(H,75,85)(H,91,92)(H4,62,63,68)(H4,64,65,69)(H4,66,67,70)/t39-,40-,41-,42-,43-,44-,45+,46+,47+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist effect against 2-methyl-5-HT activity at 5-HT3 receptor in longitudinal muscle myenteric plexus from guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50188594

(CHEBI:62878 | CHEMBL1086580)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-5-6-21-7-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50007583

(CHEBI:62882 | CHEMBL562310)Show SMILES [H][C@]12C[C@]([H])(C=C1)[C@]1([H])C(=O)N(C(=O)[C@]21[H])c1ccc(cc1)C(=O)Nc1cccc2cccnc12 |r,c:5| Show InChI InChI=1S/C25H19N3O3/c29-23(27-19-5-1-3-14-4-2-12-26-22(14)19)15-8-10-18(11-9-15)28-24(30)20-16-6-7-17(13-16)21(20)25(28)31/h1-12,16-17,20-21H,13H2,(H,27,29)/t16-,17+,20-,21+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against histamine H3 receptor in guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50408050

(CHEMBL5282944)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1cccs1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O)-c1ccccc1 Show InChI InChI=1S/C57H83N19O13S/c58-37(17-7-21-65-55(59)60)47(81)71-38(18-8-22-66-56(61)62)51(85)74-24-10-20-43(74)53(87)76-30-35(78)27-44(76)50(84)68-29-45(79)70-40(28-36-16-11-25-90-36)48(82)73-42(32-77)49(83)72-41(26-33-12-3-1-4-13-33)52(86)75(34-14-5-2-6-15-34)31-46(80)69-39(54(88)89)19-9-23-67-57(63)64/h1-6,11-16,25,35,37-44,77-78H,7-10,17-24,26-32,58H2,(H,68,84)(H,69,80)(H,70,79)(H,71,81)(H,72,83)(H,73,82)(H,88,89)(H4,59,60,65)(H4,61,62,66)(H4,63,64,67)/t35-,37+,38+,39+,40+,41-,42+,43+,44+/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against bradykinin B2 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50434157
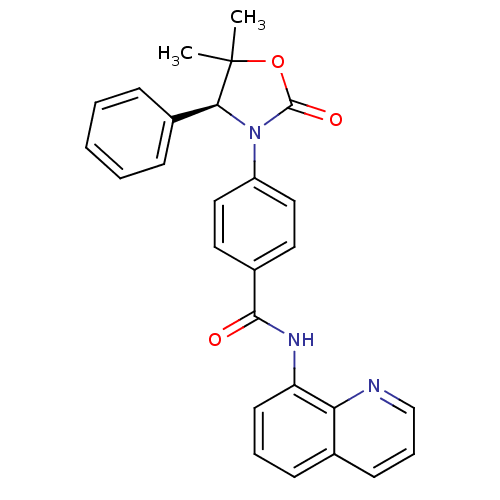
(CHEMBL2381958 | US9340549, 78)Show SMILES CC1(C)OC(=O)N([C@H]1c1ccccc1)c1ccc(cc1)C(=O)Nc1cccc2cccnc12 |r| Show InChI InChI=1S/C27H23N3O3/c1-27(2)24(19-8-4-3-5-9-19)30(26(32)33-27)21-15-13-20(14-16-21)25(31)29-22-12-6-10-18-11-7-17-28-23(18)22/h3-17,24H,1-2H3,(H,29,31)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50094941

(CHEMBL3589285)Show InChI InChI=1S/C18H15N3O/c1-20-18(22)15-12-21(11-13-7-3-2-4-8-13)16-10-6-5-9-14(16)17(15)19-20/h2-10,12H,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 423 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Catenin beta-1
(Homo sapiens (Human)) | BDBM50014132
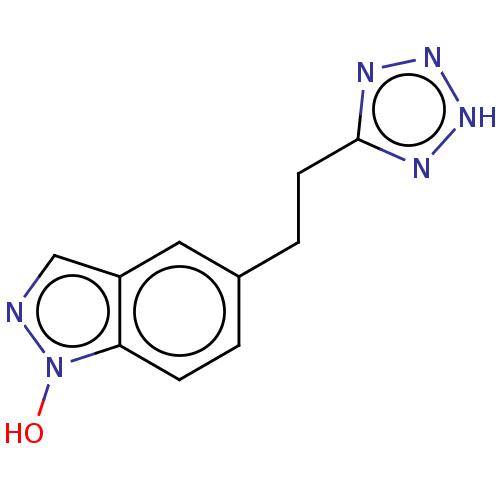
(CHEMBL2323032)Show InChI InChI=1S/C10H10N6O/c17-16-9-3-1-7(5-8(9)6-11-16)2-4-10-12-14-15-13-10/h1,3,5-6,17H,2,4H2,(H,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50380592

(CHEMBL1552719)Show SMILES COc1ccc(cc1)-n1c(SCc2nc(no2)-c2ccc(C)cc2)nnc1-c1ccncc1 Show InChI InChI=1S/C24H20N6O2S/c1-16-3-5-17(6-4-16)22-26-21(32-29-22)15-33-24-28-27-23(18-11-13-25-14-12-18)30(24)19-7-9-20(31-2)10-8-19/h3-14H,15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Uterotonic activity against OT receptor in Wistar rat in absence of magnesium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50505333

(CHEMBL4437173)Show SMILES CC(C)(O)c1ccc(cc1)-c1nc2c(F)cc(F)cc2c(=O)[nH]1 Show InChI InChI=1S/C17H14F2N2O2/c1-17(2,23)10-5-3-9(4-6-10)15-20-14-12(16(22)21-15)7-11(18)8-13(14)19/h3-8,23H,1-2H3,(H,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroidogenic factor 1
(Homo sapiens (Human)) | BDBM50380592

(CHEMBL1552719)Show SMILES COc1ccc(cc1)-n1c(SCc2nc(no2)-c2ccc(C)cc2)nnc1-c1ccncc1 Show InChI InChI=1S/C24H20N6O2S/c1-16-3-5-17(6-4-16)22-26-21(32-29-22)15-33-24-28-27-23(18-11-13-25-14-12-18)30(24)19-7-9-20(31-2)10-8-19/h3-14H,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Uterotonic activity against OT receptor in Wistar rat in absence of magnesium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50136777
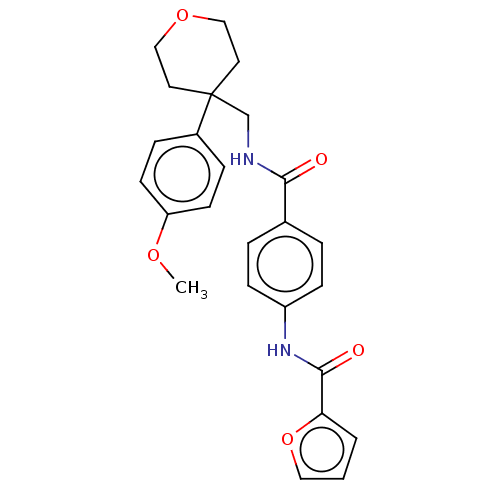
(CHEMBL3753077)Show SMILES COc1ccc(cc1)C1(CNC(=O)c2ccc(NC(=O)c3ccco3)cc2)CCOCC1 Show InChI InChI=1S/C25H26N2O5/c1-30-21-10-6-19(7-11-21)25(12-15-31-16-13-25)17-26-23(28)18-4-8-20(9-5-18)27-24(29)22-3-2-14-32-22/h2-11,14H,12-13,15-17H2,1H3,(H,26,28)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effect on IP3 turnover by phospholipaseC positively linked to human NK1 receptor expressed in CHO cells |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sulfotransferase 2A1
(Homo sapiens (Human)) | BDBM50427989
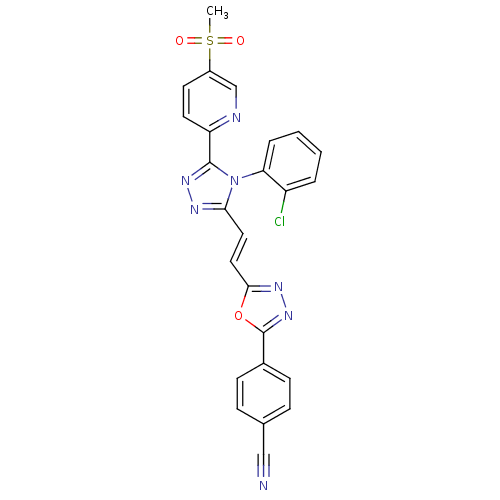
(CHEMBL2325503)Show SMILES CS(=O)(=O)c1ccc(nc1)-c1nnc(\C=C\c2nnc(o2)-c2ccc(cc2)C#N)n1-c1ccccc1Cl |(62.04,-5.04,;60.89,-4.01,;60.11,-2.67,;61.66,-2.66,;59.43,-4.48,;58.27,-3.45,;56.81,-3.93,;56.5,-5.43,;57.64,-6.47,;59.11,-5.99,;55.04,-5.91,;54.56,-7.37,;53.02,-7.37,;52.55,-5.91,;51.08,-5.43,;49.94,-6.46,;48.47,-5.99,;48,-4.52,;46.46,-4.52,;45.98,-5.98,;47.23,-6.89,;44.52,-6.46,;43.37,-5.43,;41.92,-5.9,;41.6,-7.41,;42.74,-8.44,;44.2,-7.96,;40.13,-7.89,;38.67,-8.37,;53.79,-5,;53.8,-3.47,;55.13,-2.69,;55.13,-1.14,;53.79,-.38,;52.46,-1.15,;52.46,-2.7,;51.13,-3.46,)| Show InChI InChI=1S/C25H16ClN7O3S/c1-37(34,35)18-10-11-20(28-15-18)24-31-29-22(33(24)21-5-3-2-4-19(21)26)12-13-23-30-32-25(36-23)17-8-6-16(14-27)7-9-17/h2-13,15H,1H3/b13-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data