 Found 153 hits Enz. Inhib. hit(s) with all data for entry = 50018450
Found 153 hits Enz. Inhib. hit(s) with all data for entry = 50018450 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195903
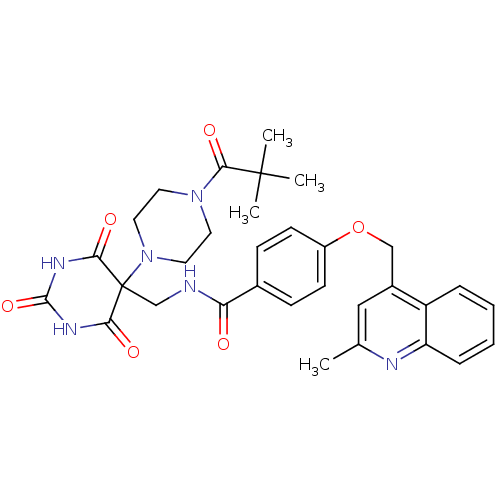
(4-((2-methylquinolin-4-yl)methoxy)-N-((2,4,6-triox...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(CC3)C(=O)C(C)(C)C)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C32H36N6O6/c1-20-17-22(24-7-5-6-8-25(24)34-20)18-44-23-11-9-21(10-12-23)26(39)33-19-32(27(40)35-30(43)36-28(32)41)38-15-13-37(14-16-38)29(42)31(2,3)4/h5-12,17H,13-16,18-19H2,1-4H3,(H,33,39)(H2,35,36,40,41,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195903

(4-((2-methylquinolin-4-yl)methoxy)-N-((2,4,6-triox...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(CC3)C(=O)C(C)(C)C)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C32H36N6O6/c1-20-17-22(24-7-5-6-8-25(24)34-20)18-44-23-11-9-21(10-12-23)26(39)33-19-32(27(40)35-30(43)36-28(32)41)38-15-13-37(14-16-38)29(42)31(2,3)4/h5-12,17H,13-16,18-19H2,1-4H3,(H,33,39)(H2,35,36,40,41,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195904
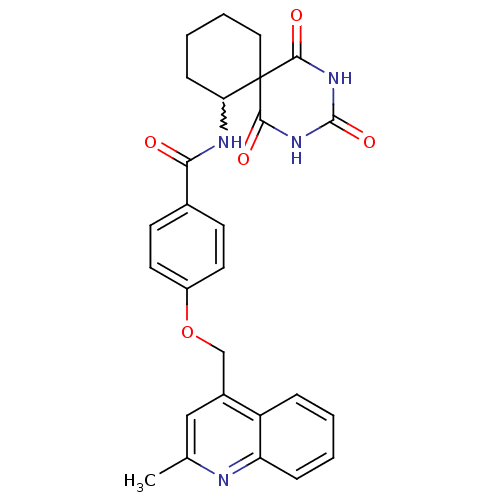
(4-(2-methyl-quinolin-4-ylmethoxy)-N-(1,3,5-trioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CCCCC22C(=O)NC(=O)NC2=O)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C27H26N4O5/c1-16-14-18(20-6-2-3-7-21(20)28-16)15-36-19-11-9-17(10-12-19)23(32)29-22-8-4-5-13-27(22)24(33)30-26(35)31-25(27)34/h2-3,6-7,9-12,14,22H,4-5,8,13,15H2,1H3,(H,29,32)(H2,30,31,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195917
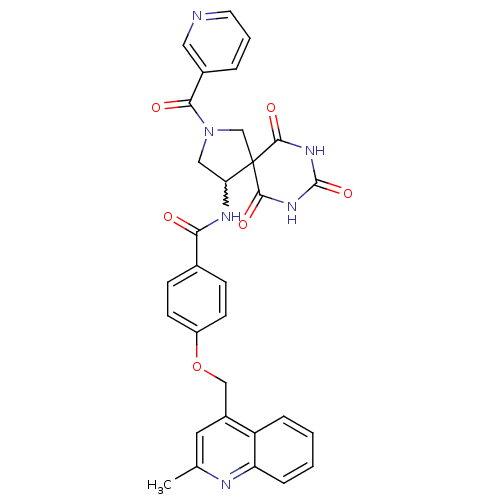
(4-(2-methyl-quinolin-4-ylmethoxy)-N-[6,8,10-trioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)C(=O)c2cccnc2)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C31H26N6O6/c1-18-13-21(23-6-2-3-7-24(23)33-18)16-43-22-10-8-19(9-11-22)26(38)34-25-15-37(27(39)20-5-4-12-32-14-20)17-31(25)28(40)35-30(42)36-29(31)41/h2-14,25H,15-17H2,1H3,(H,34,38)(H2,35,36,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195905

(CHEMBL240464 | N-((5-ethyl-2,4,6-trioxo-hexahydrop...)Show SMILES CCC1(CNC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C25H24N4O5/c1-3-25(22(31)28-24(33)29-23(25)32)14-26-21(30)16-8-10-18(11-9-16)34-13-17-12-15(2)27-20-7-5-4-6-19(17)20/h4-12H,3,13-14H2,1-2H3,(H,26,30)(H2,28,29,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195905

(CHEMBL240464 | N-((5-ethyl-2,4,6-trioxo-hexahydrop...)Show SMILES CCC1(CNC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C25H24N4O5/c1-3-25(22(31)28-24(33)29-23(25)32)14-26-21(30)16-8-10-18(11-9-16)34-13-17-12-15(2)27-20-7-5-4-6-19(17)20/h4-12H,3,13-14H2,1-2H3,(H,26,30)(H2,28,29,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195899
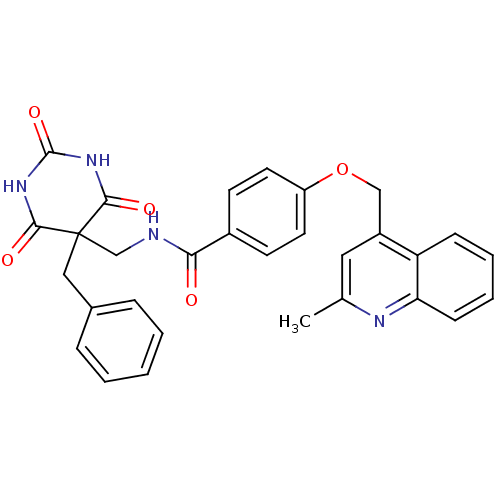
(CHEMBL440048 | N-((5-benzyl-2,4,6-trioxo-hexahydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(Cc3ccccc3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C30H26N4O5/c1-19-15-22(24-9-5-6-10-25(24)32-19)17-39-23-13-11-21(12-14-23)26(35)31-18-30(16-20-7-3-2-4-8-20)27(36)33-29(38)34-28(30)37/h2-15H,16-18H2,1H3,(H,31,35)(H2,33,34,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195899

(CHEMBL440048 | N-((5-benzyl-2,4,6-trioxo-hexahydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(Cc3ccccc3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C30H26N4O5/c1-19-15-22(24-9-5-6-10-25(24)32-19)17-39-23-13-11-21(12-14-23)26(35)31-18-30(16-20-7-3-2-4-8-20)27(36)33-29(38)34-28(30)37/h2-15H,16-18H2,1H3,(H,31,35)(H2,33,34,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195912
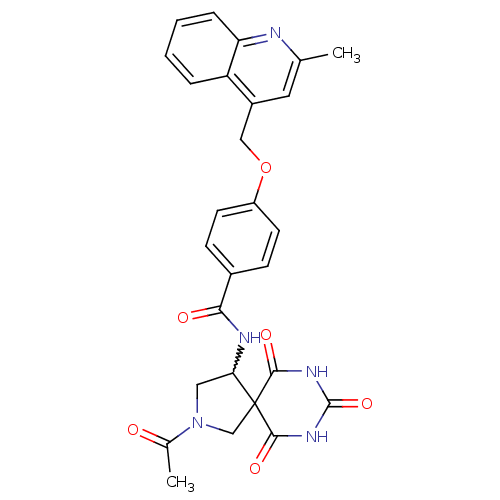
(CHEMBL248173 | N-(2-acetyl-6,8,10-trioxo-2,7,9-tri...)Show SMILES CC(=O)N1CC(NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C2(C1)C(=O)NC(=O)NC2=O |w:5.5| Show InChI InChI=1S/C27H25N5O6/c1-15-11-18(20-5-3-4-6-21(20)28-15)13-38-19-9-7-17(8-10-19)23(34)29-22-12-32(16(2)33)14-27(22)24(35)30-26(37)31-25(27)36/h3-11,22H,12-14H2,1-2H3,(H,29,34)(H2,30,31,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195916
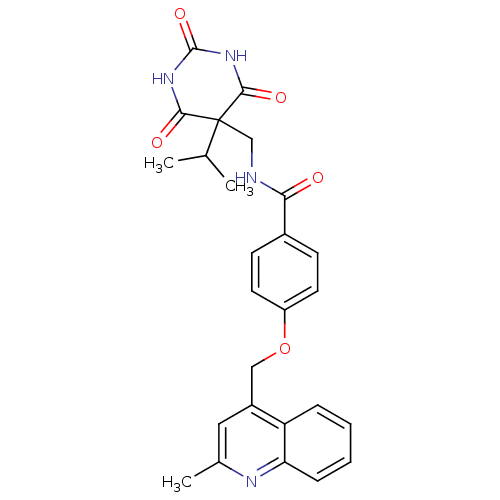
(CHEMBL240653 | N-((5-isopropyl-2,4,6-trioxo-hexahy...)Show SMILES CC(C)C1(CNC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C26H26N4O5/c1-15(2)26(23(32)29-25(34)30-24(26)33)14-27-22(31)17-8-10-19(11-9-17)35-13-18-12-16(3)28-21-7-5-4-6-20(18)21/h4-12,15H,13-14H2,1-3H3,(H,27,31)(H2,29,30,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195898
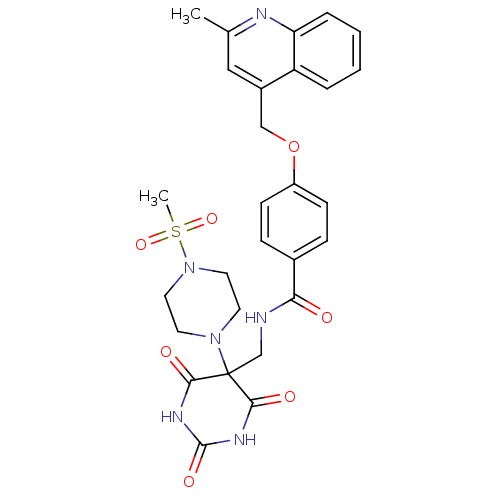
(4-((2-methylquinolin-4-yl)methoxy)-N-((5-(4-(methy...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(CC3)S(C)(=O)=O)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C28H30N6O7S/c1-18-15-20(22-5-3-4-6-23(22)30-18)16-41-21-9-7-19(8-10-21)24(35)29-17-28(25(36)31-27(38)32-26(28)37)33-11-13-34(14-12-33)42(2,39)40/h3-10,15H,11-14,16-17H2,1-2H3,(H,29,35)(H2,31,32,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195897
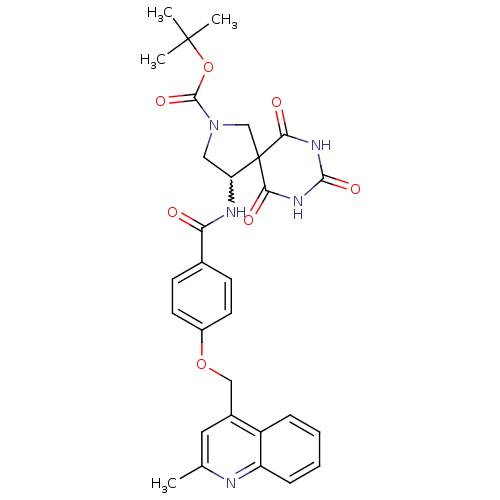
(4-[4-(2-methyl-quinolin-4-ylmethoxy)-benzoylamino]...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)C(=O)OC(C)(C)C)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C30H31N5O7/c1-17-13-19(21-7-5-6-8-22(21)31-17)15-41-20-11-9-18(10-12-20)24(36)32-23-14-35(28(40)42-29(2,3)4)16-30(23)25(37)33-27(39)34-26(30)38/h5-13,23H,14-16H2,1-4H3,(H,32,36)(H2,33,34,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195921
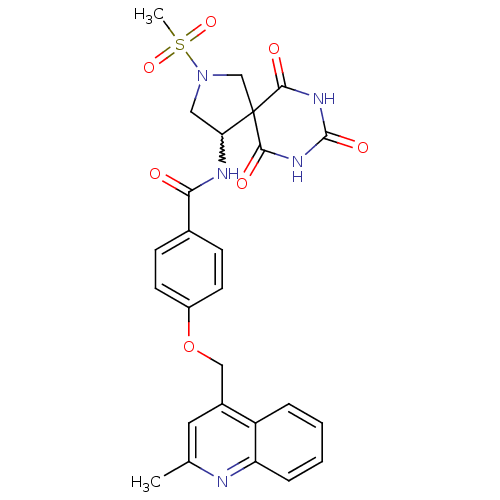
(CHEMBL429112 | N-(2-methanesulfonyl-6,8,10-trioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)S(C)(=O)=O)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C26H25N5O7S/c1-15-11-17(19-5-3-4-6-20(19)27-15)13-38-18-9-7-16(8-10-18)22(32)28-21-12-31(39(2,36)37)14-26(21)23(33)29-25(35)30-24(26)34/h3-11,21H,12-14H2,1-2H3,(H,28,32)(H2,29,30,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50195917

(4-(2-methyl-quinolin-4-ylmethoxy)-N-[6,8,10-trioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)C(=O)c2cccnc2)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C31H26N6O6/c1-18-13-21(23-6-2-3-7-24(23)33-18)16-43-22-10-8-19(9-11-22)26(38)34-25-15-37(27(39)20-5-4-12-32-14-20)17-31(25)28(40)35-30(42)36-29(31)41/h2-14,25H,15-17H2,1H3,(H,34,38)(H2,35,36,40,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50195906
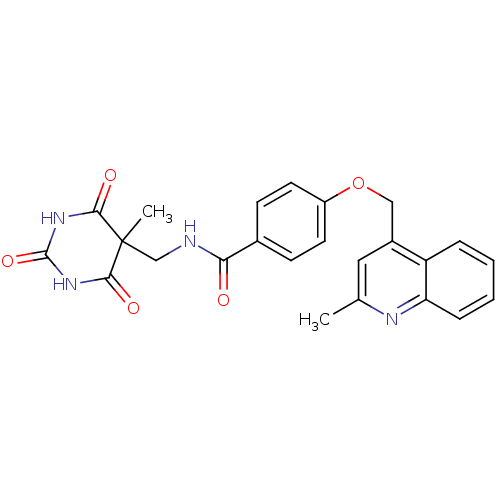
(CHEMBL241048 | N-((5-methyl-2,4,6-trioxo-hexahydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(C)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C24H22N4O5/c1-14-11-16(18-5-3-4-6-19(18)26-14)12-33-17-9-7-15(8-10-17)20(29)25-13-24(2)21(30)27-23(32)28-22(24)31/h3-11H,12-13H2,1-2H3,(H,25,29)(H2,27,28,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195898

(4-((2-methylquinolin-4-yl)methoxy)-N-((5-(4-(methy...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(CC3)S(C)(=O)=O)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C28H30N6O7S/c1-18-15-20(22-5-3-4-6-23(22)30-18)16-41-21-9-7-19(8-10-21)24(35)29-17-28(25(36)31-27(38)32-26(28)37)33-11-13-34(14-12-33)42(2,39)40/h3-10,15H,11-14,16-17H2,1-2H3,(H,29,35)(H2,31,32,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195920
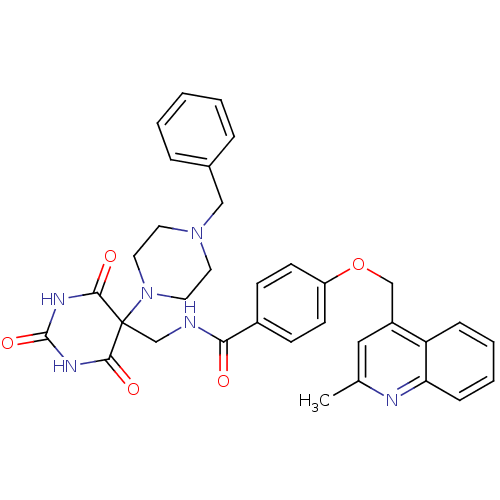
(CHEMBL240854 | N-((5-(4-benzylpiperazin-1-yl)-2,4,...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(Cc4ccccc4)CC3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C34H34N6O5/c1-23-19-26(28-9-5-6-10-29(28)36-23)21-45-27-13-11-25(12-14-27)30(41)35-22-34(31(42)37-33(44)38-32(34)43)40-17-15-39(16-18-40)20-24-7-3-2-4-8-24/h2-14,19H,15-18,20-22H2,1H3,(H,35,41)(H2,37,38,42,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195896
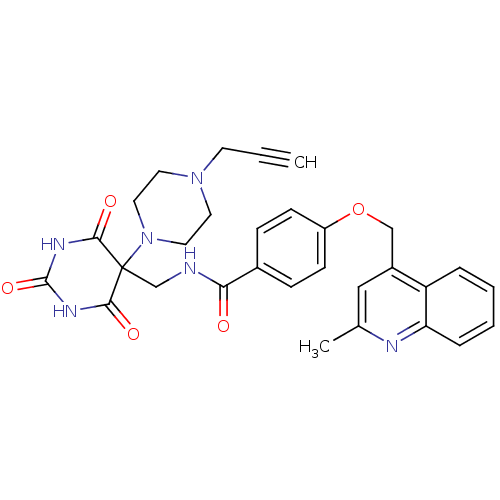
(4-((2-methylquinolin-4-yl)methoxy)-N-((2,4,6-triox...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(CC#C)CC3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C30H30N6O5/c1-3-12-35-13-15-36(16-14-35)30(27(38)33-29(40)34-28(30)39)19-31-26(37)21-8-10-23(11-9-21)41-18-22-17-20(2)32-25-7-5-4-6-24(22)25/h1,4-11,17H,12-16,18-19H2,2H3,(H,31,37)(H2,33,34,38,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195902
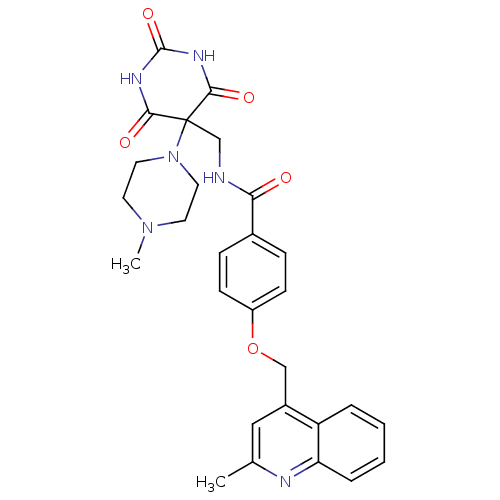
(CHEMBL428212 | N-((5-(4-methylpiperazin-1-yl)-2,4,...)Show SMILES CN1CCN(CC1)C1(CNC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C28H30N6O5/c1-18-15-20(22-5-3-4-6-23(22)30-18)16-39-21-9-7-19(8-10-21)24(35)29-17-28(34-13-11-33(2)12-14-34)25(36)31-27(38)32-26(28)37/h3-10,15H,11-14,16-17H2,1-2H3,(H,29,35)(H2,31,32,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195897

(4-[4-(2-methyl-quinolin-4-ylmethoxy)-benzoylamino]...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)C(=O)OC(C)(C)C)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C30H31N5O7/c1-17-13-19(21-7-5-6-8-22(21)31-17)15-41-20-11-9-18(10-12-20)24(36)32-23-14-35(28(40)42-29(2,3)4)16-30(23)25(37)33-27(39)34-26(30)38/h5-13,23H,14-16H2,1-4H3,(H,32,36)(H2,33,34,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195908
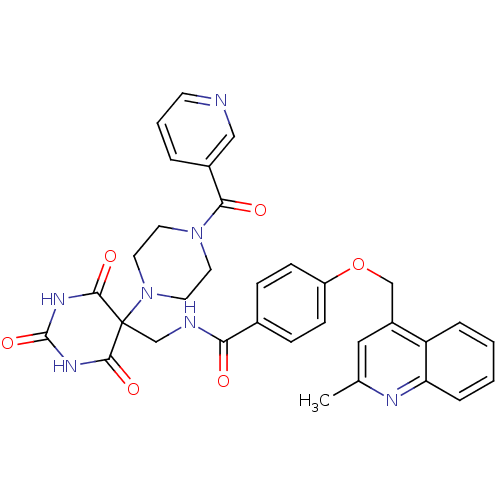
(4-((2-methylquinolin-4-yl)methoxy)-N-((5-(4-nicoti...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(CC3)C(=O)c3cccnc3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C33H31N7O6/c1-21-17-24(26-6-2-3-7-27(26)36-21)19-46-25-10-8-22(9-11-25)28(41)35-20-33(30(43)37-32(45)38-31(33)44)40-15-13-39(14-16-40)29(42)23-5-4-12-34-18-23/h2-12,17-18H,13-16,19-20H2,1H3,(H,35,41)(H2,37,38,43,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195914
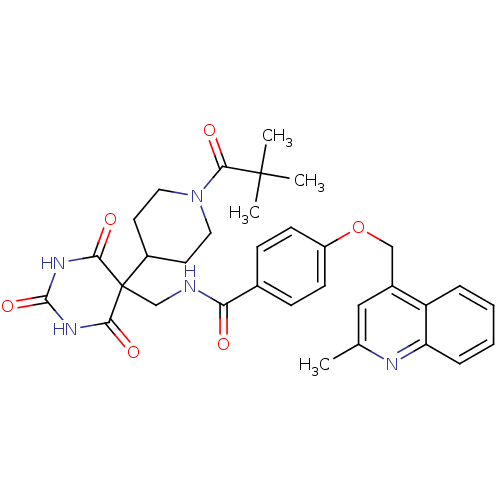
(4-((2-methylquinolin-4-yl)methoxy)-N-((2,4,6-triox...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(C3CCN(CC3)C(=O)C(C)(C)C)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C33H37N5O6/c1-20-17-22(25-7-5-6-8-26(25)35-20)18-44-24-11-9-21(10-12-24)27(39)34-19-33(28(40)36-31(43)37-29(33)41)23-13-15-38(16-14-23)30(42)32(2,3)4/h5-12,17,23H,13-16,18-19H2,1-4H3,(H,34,39)(H2,36,37,40,41,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195928
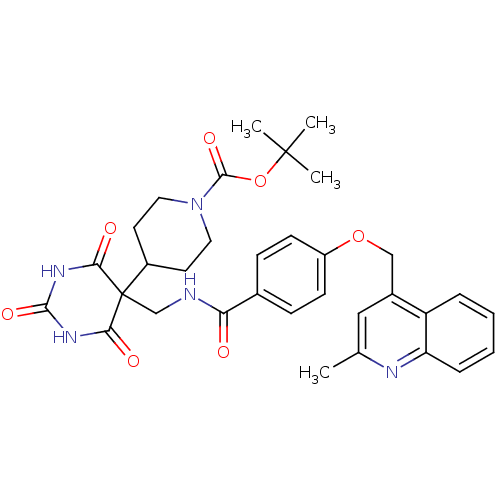
(CHEMBL240652 | tert-butyl 4-(5-((4-((2-methylquino...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(C3CCN(CC3)C(=O)OC(C)(C)C)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C33H37N5O7/c1-20-17-22(25-7-5-6-8-26(25)35-20)18-44-24-11-9-21(10-12-24)27(39)34-19-33(28(40)36-30(42)37-29(33)41)23-13-15-38(16-14-23)31(43)45-32(2,3)4/h5-12,17,23H,13-16,18-19H2,1-4H3,(H,34,39)(H2,36,37,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195907
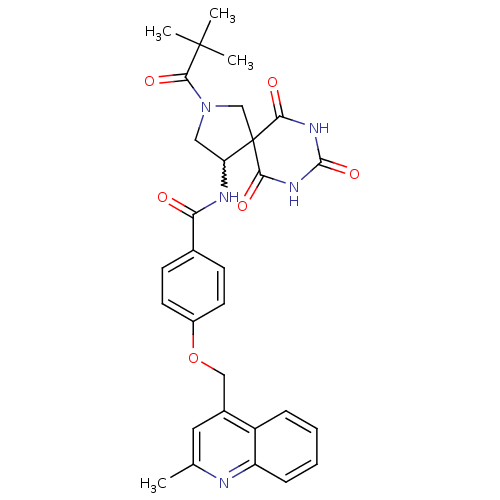
(CHEMBL392867 | N-[2-(2,2-dimethyl-propionyl)-6,8,1...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)C(=O)C(C)(C)C)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C30H31N5O6/c1-17-13-19(21-7-5-6-8-22(21)31-17)15-41-20-11-9-18(10-12-20)24(36)32-23-14-35(27(39)29(2,3)4)16-30(23)25(37)33-28(40)34-26(30)38/h5-13,23H,14-16H2,1-4H3,(H,32,36)(H2,33,34,37,38,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195915
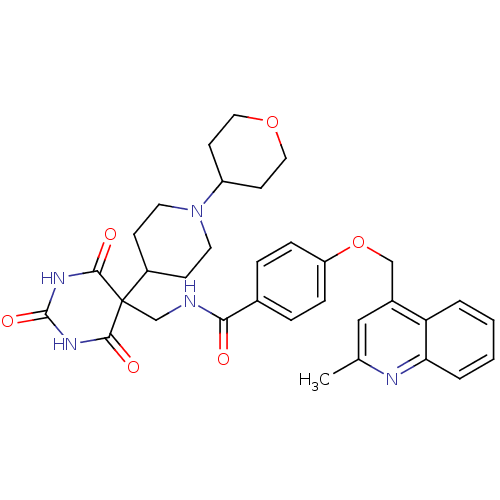
(4-((2-methylquinolin-4-yl)methoxy)-N-((2,4,6-triox...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(C3CCN(CC3)C3CCOCC3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C33H37N5O6/c1-21-18-23(27-4-2-3-5-28(27)35-21)19-44-26-8-6-22(7-9-26)29(39)34-20-33(30(40)36-32(42)37-31(33)41)24-10-14-38(15-11-24)25-12-16-43-17-13-25/h2-9,18,24-25H,10-17,19-20H2,1H3,(H,34,39)(H2,36,37,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195910
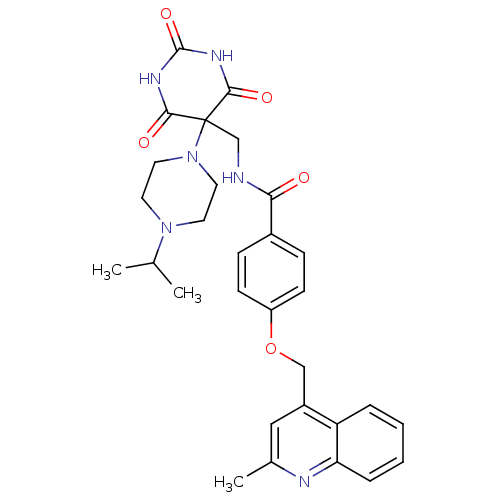
(CHEMBL241051 | N-((5-(4-isopropylpiperazin-1-yl)-2...)Show SMILES CC(C)N1CCN(CC1)C1(CNC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C30H34N6O5/c1-19(2)35-12-14-36(15-13-35)30(27(38)33-29(40)34-28(30)39)18-31-26(37)21-8-10-23(11-9-21)41-17-22-16-20(3)32-25-7-5-4-6-24(22)25/h4-11,16,19H,12-15,17-18H2,1-3H3,(H,31,37)(H2,33,34,38,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195909
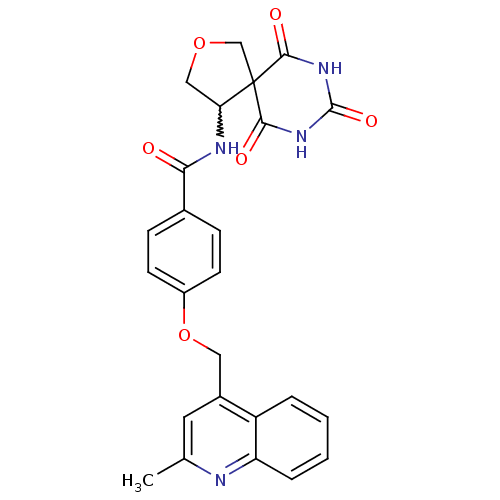
(4-(2-methyl-quinolin-4-ylmethoxy)-N-(6,8,10-trioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2COCC22C(=O)NC(=O)NC2=O)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C25H22N4O6/c1-14-10-16(18-4-2-3-5-19(18)26-14)11-35-17-8-6-15(7-9-17)21(30)27-20-12-34-13-25(20)22(31)28-24(33)29-23(25)32/h2-10,20H,11-13H2,1H3,(H,27,30)(H2,28,29,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195900
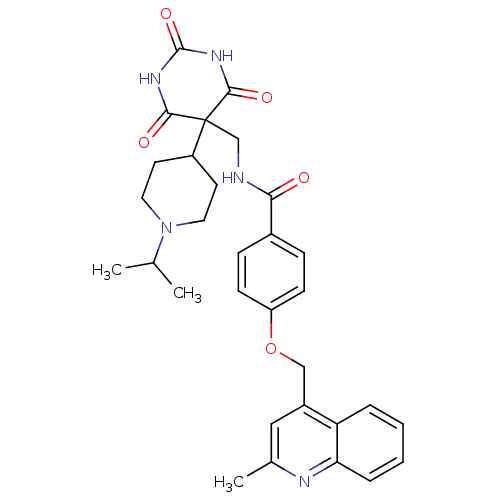
(CHEMBL438125 | N-((5-(1-isopropylpiperidin-4-yl)-2...)Show SMILES CC(C)N1CCC(CC1)C1(CNC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C31H35N5O5/c1-19(2)36-14-12-23(13-15-36)31(28(38)34-30(40)35-29(31)39)18-32-27(37)21-8-10-24(11-9-21)41-17-22-16-20(3)33-26-7-5-4-6-25(22)26/h4-11,16,19,23H,12-15,17-18H2,1-3H3,(H,32,37)(H2,34,35,38,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195923
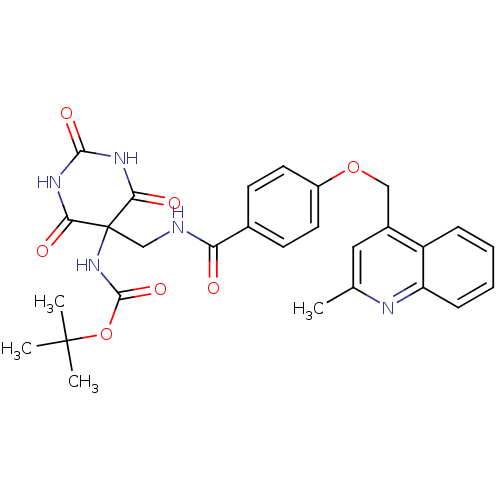
(CHEMBL427861 | tert-butyl 5-((4-((2-methylquinolin...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(NC(=O)OC(C)(C)C)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C28H29N5O7/c1-16-13-18(20-7-5-6-8-21(20)30-16)14-39-19-11-9-17(10-12-19)22(34)29-15-28(33-26(38)40-27(2,3)4)23(35)31-25(37)32-24(28)36/h5-13H,14-15H2,1-4H3,(H,29,34)(H,33,38)(H2,31,32,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195895
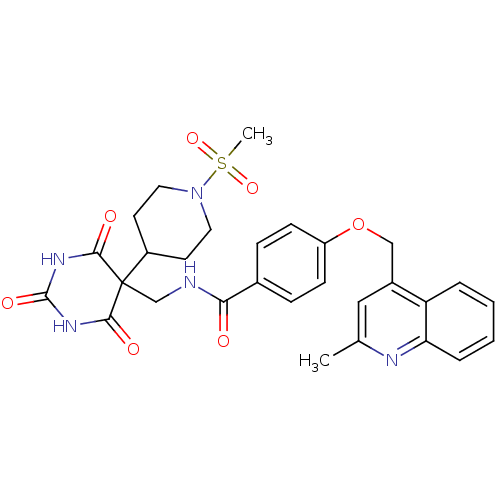
(4-((2-methylquinolin-4-yl)methoxy)-N-((5-(1-(methy...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(C3CCN(CC3)S(C)(=O)=O)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C29H31N5O7S/c1-18-15-20(23-5-3-4-6-24(23)31-18)16-41-22-9-7-19(8-10-22)25(35)30-17-29(26(36)32-28(38)33-27(29)37)21-11-13-34(14-12-21)42(2,39)40/h3-10,15,21H,11-14,16-17H2,1-2H3,(H,30,35)(H2,32,33,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195913
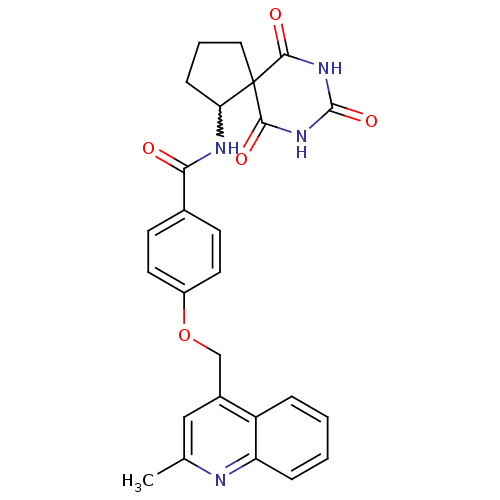
(4-(2-methyl-quinolin-4-ylmethoxy)-N-(6,8,10-trioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CCCC22C(=O)NC(=O)NC2=O)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C26H24N4O5/c1-15-13-17(19-5-2-3-6-20(19)27-15)14-35-18-10-8-16(9-11-18)22(31)28-21-7-4-12-26(21)23(32)29-25(34)30-24(26)33/h2-3,5-6,8-11,13,21H,4,7,12,14H2,1H3,(H,28,31)(H2,29,30,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195921

(CHEMBL429112 | N-(2-methanesulfonyl-6,8,10-trioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)S(C)(=O)=O)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C26H25N5O7S/c1-15-11-17(19-5-3-4-6-20(19)27-15)13-38-18-9-7-16(8-10-18)22(32)28-21-12-31(39(2,36)37)14-26(21)23(33)29-25(35)30-24(26)34/h3-11,21H,12-14H2,1-2H3,(H,28,32)(H2,29,30,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195924
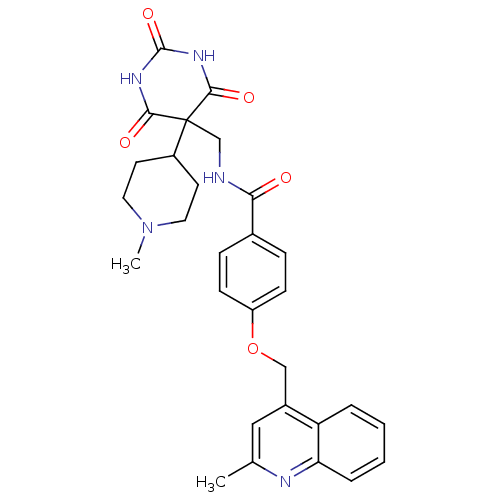
(CHEMBL240852 | N-((5-(1-methylpiperidin-4-yl)-2,4,...)Show SMILES CN1CCC(CC1)C1(CNC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C29H31N5O5/c1-18-15-20(23-5-3-4-6-24(23)31-18)16-39-22-9-7-19(8-10-22)25(35)30-17-29(21-11-13-34(2)14-12-21)26(36)32-28(38)33-27(29)37/h3-10,15,21H,11-14,16-17H2,1-2H3,(H,30,35)(H2,32,33,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195912

(CHEMBL248173 | N-(2-acetyl-6,8,10-trioxo-2,7,9-tri...)Show SMILES CC(=O)N1CC(NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C2(C1)C(=O)NC(=O)NC2=O |w:5.5| Show InChI InChI=1S/C27H25N5O6/c1-15-11-18(20-5-3-4-6-21(20)28-15)13-38-19-9-7-17(8-10-19)23(34)29-22-12-32(16(2)33)14-27(22)24(35)30-26(37)31-25(27)36/h3-11,22H,12-14H2,1-2H3,(H,29,34)(H2,30,31,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195906

(CHEMBL241048 | N-((5-methyl-2,4,6-trioxo-hexahydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(C)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C24H22N4O5/c1-14-11-16(18-5-3-4-6-19(18)26-14)12-33-17-9-7-15(8-10-17)20(29)25-13-24(2)21(30)27-23(32)28-22(24)31/h3-11H,12-13H2,1-2H3,(H,25,29)(H2,27,28,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195911
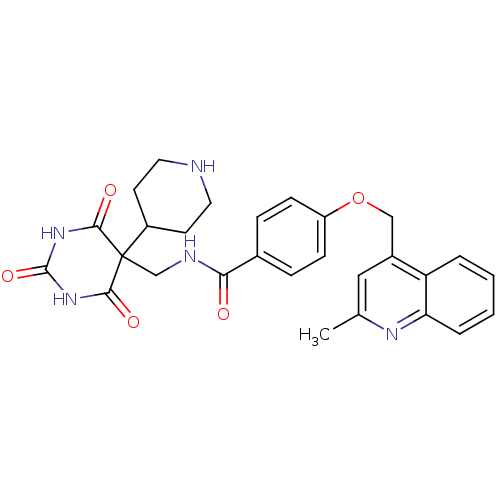
(4-((2-methylquinolin-4-yl)methoxy)-N-((2,4,6-triox...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(C3CCNCC3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C28H29N5O5/c1-17-14-19(22-4-2-3-5-23(22)31-17)15-38-21-8-6-18(7-9-21)24(34)30-16-28(20-10-12-29-13-11-20)25(35)32-27(37)33-26(28)36/h2-9,14,20,29H,10-13,15-16H2,1H3,(H,30,34)(H2,32,33,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195901
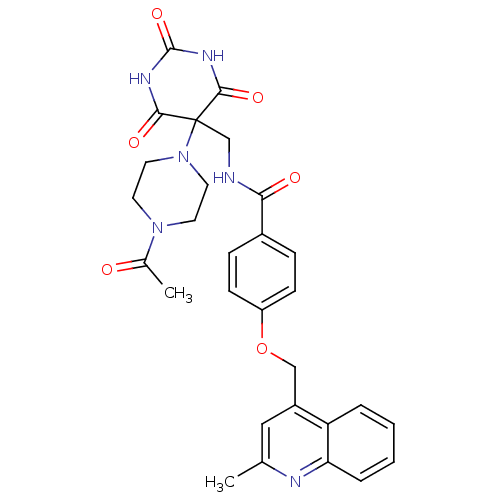
(CHEMBL247966 | N-((5-(4-acetylpiperazin-1-yl)-2,4,...)Show SMILES CC(=O)N1CCN(CC1)C1(CNC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C29H30N6O6/c1-18-15-21(23-5-3-4-6-24(23)31-18)16-41-22-9-7-20(8-10-22)25(37)30-17-29(26(38)32-28(40)33-27(29)39)35-13-11-34(12-14-35)19(2)36/h3-10,15H,11-14,16-17H2,1-2H3,(H,30,37)(H2,32,33,38,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50195917

(4-(2-methyl-quinolin-4-ylmethoxy)-N-[6,8,10-trioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)C(=O)c2cccnc2)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C31H26N6O6/c1-18-13-21(23-6-2-3-7-24(23)33-18)16-43-22-10-8-19(9-11-22)26(38)34-25-15-37(27(39)20-5-4-12-32-14-20)17-31(25)28(40)35-30(42)36-29(31)41/h2-14,25H,15-17H2,1H3,(H,34,38)(H2,35,36,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50195909

(4-(2-methyl-quinolin-4-ylmethoxy)-N-(6,8,10-trioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2COCC22C(=O)NC(=O)NC2=O)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C25H22N4O6/c1-14-10-16(18-4-2-3-5-19(18)26-14)11-35-17-8-6-15(7-9-17)21(30)27-20-12-34-13-25(20)22(31)28-24(33)29-23(25)32/h2-10,20H,11-13H2,1H3,(H,27,30)(H2,28,29,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50195899

(CHEMBL440048 | N-((5-benzyl-2,4,6-trioxo-hexahydro...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(Cc3ccccc3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C30H26N4O5/c1-19-15-22(24-9-5-6-10-25(24)32-19)17-39-23-13-11-21(12-14-23)26(35)31-18-30(16-20-7-3-2-4-8-20)27(36)33-29(38)34-28(30)37/h2-15H,16-18H2,1H3,(H,31,35)(H2,33,34,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50195921

(CHEMBL429112 | N-(2-methanesulfonyl-6,8,10-trioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)S(C)(=O)=O)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C26H25N5O7S/c1-15-11-17(19-5-3-4-6-20(19)27-15)13-38-18-9-7-16(8-10-18)22(32)28-21-12-31(39(2,36)37)14-26(21)23(33)29-25(35)30-24(26)34/h3-11,21H,12-14H2,1-2H3,(H,28,32)(H2,29,30,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50195912

(CHEMBL248173 | N-(2-acetyl-6,8,10-trioxo-2,7,9-tri...)Show SMILES CC(=O)N1CC(NC(=O)c2ccc(OCc3cc(C)nc4ccccc34)cc2)C2(C1)C(=O)NC(=O)NC2=O |w:5.5| Show InChI InChI=1S/C27H25N5O6/c1-15-11-18(20-5-3-4-6-21(20)28-15)13-38-19-9-7-17(8-10-19)23(34)29-22-12-32(16(2)33)14-27(22)24(35)30-26(37)31-25(27)36/h3-11,22H,12-14H2,1-2H3,(H,29,34)(H2,30,31,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195909

(4-(2-methyl-quinolin-4-ylmethoxy)-N-(6,8,10-trioxo...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2COCC22C(=O)NC(=O)NC2=O)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C25H22N4O6/c1-14-10-16(18-4-2-3-5-19(18)26-14)11-35-17-8-6-15(7-9-17)21(30)27-20-12-34-13-25(20)22(31)28-24(33)29-23(25)32/h2-10,20H,11-13H2,1H3,(H,27,30)(H2,28,29,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50195920

(CHEMBL240854 | N-((5-(4-benzylpiperazin-1-yl)-2,4,...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(Cc4ccccc4)CC3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C34H34N6O5/c1-23-19-26(28-9-5-6-10-29(28)36-23)21-45-27-13-11-25(12-14-27)30(41)35-22-34(31(42)37-33(44)38-32(34)43)40-17-15-39(16-18-40)20-24-7-3-2-4-8-24/h2-14,19H,15-18,20-22H2,1H3,(H,35,41)(H2,37,38,42,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50195921

(CHEMBL429112 | N-(2-methanesulfonyl-6,8,10-trioxo-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)S(C)(=O)=O)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C26H25N5O7S/c1-15-11-17(19-5-3-4-6-20(19)27-15)13-38-18-9-7-16(8-10-18)22(32)28-21-12-31(39(2,36)37)14-26(21)23(33)29-25(35)30-24(26)34/h3-11,21H,12-14H2,1-2H3,(H,28,32)(H2,29,30,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50195915

(4-((2-methylquinolin-4-yl)methoxy)-N-((2,4,6-triox...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(C3CCN(CC3)C3CCOCC3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C33H37N5O6/c1-21-18-23(27-4-2-3-5-28(27)35-21)19-44-26-8-6-22(7-9-26)29(39)34-20-33(30(40)36-32(42)37-31(33)41)24-10-14-38(15-11-24)25-12-16-43-17-13-25/h2-9,18,24-25H,10-17,19-20H2,1H3,(H,34,39)(H2,36,37,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50195911

(4-((2-methylquinolin-4-yl)methoxy)-N-((2,4,6-triox...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(C3CCNCC3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C28H29N5O5/c1-17-14-19(22-4-2-3-5-23(22)31-17)15-38-21-8-6-18(7-9-21)24(34)30-16-28(20-10-12-29-13-11-20)25(35)32-27(37)33-26(28)36/h2-9,14,20,29H,10-13,15-16H2,1H3,(H,30,34)(H2,32,33,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50195920

(CHEMBL240854 | N-((5-(4-benzylpiperazin-1-yl)-2,4,...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(Cc4ccccc4)CC3)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C34H34N6O5/c1-23-19-26(28-9-5-6-10-29(28)36-23)21-45-27-13-11-25(12-14-27)30(41)35-22-34(31(42)37-33(44)38-32(34)43)40-17-15-39(16-18-40)20-24-7-3-2-4-8-24/h2-14,19H,15-18,20-22H2,1H3,(H,35,41)(H2,37,38,42,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50195898

(4-((2-methylquinolin-4-yl)methoxy)-N-((5-(4-(methy...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NCC2(N3CCN(CC3)S(C)(=O)=O)C(=O)NC(=O)NC2=O)c2ccccc2n1 Show InChI InChI=1S/C28H30N6O7S/c1-18-15-20(22-5-3-4-6-23(22)30-18)16-41-21-9-7-19(8-10-21)24(35)29-17-28(25(36)31-27(38)32-26(28)37)33-11-13-34(14-12-33)42(2,39)40/h3-10,15H,11-14,16-17H2,1-2H3,(H,29,35)(H2,31,32,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50195907

(CHEMBL392867 | N-[2-(2,2-dimethyl-propionyl)-6,8,1...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2CN(CC22C(=O)NC(=O)NC2=O)C(=O)C(C)(C)C)c2ccccc2n1 |w:15.15| Show InChI InChI=1S/C30H31N5O6/c1-17-13-19(21-7-5-6-8-22(21)31-17)15-41-20-11-9-18(10-12-20)24(36)32-23-14-35(27(39)29(2,3)4)16-30(23)25(37)33-28(40)34-26(30)38/h5-13,23H,14-16H2,1-4H3,(H,32,36)(H2,33,34,37,38,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 266-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.048
BindingDB Entry DOI: 10.7270/Q2NK3DPF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data