 Found 98 hits Enz. Inhib. hit(s) with all data for entry = 50018988
Found 98 hits Enz. Inhib. hit(s) with all data for entry = 50018988 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50614466
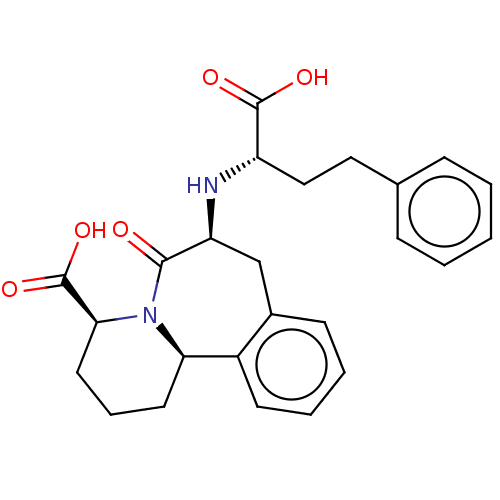
(CHEMBL5272537)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)N[C@@H](CCc1ccccc1)C(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50103430
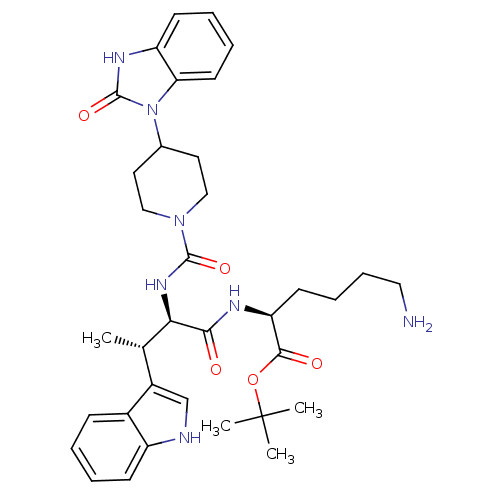
(6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50614463

(CHEMBL5283332)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)NC(=O)[C@H](S)Cc1ccccc1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50614462

(CHEMBL5277842)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)NC(=O)[C@@H](S)Cc1ccccc1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50614462

(CHEMBL5277842)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)NC(=O)[C@@H](S)Cc1ccccc1)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50614474

(CHEMBL5274928)Show SMILES [H][C@@]12CCC[C@]1([H])N([C@@H](C2)C(=O)NC(CCCNC(N)=N)C(O)=O)C(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@H](CO)NC(=O)[C@H](Cc1cccs1)NC(=O)CNC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](N)CCCNC(N)=N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50614476

(CHEMBL5280457)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1C[C@H](O)C[C@H]1C(=O)NCC(=O)N[C@@H](Cc1cccs1)C(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50614468
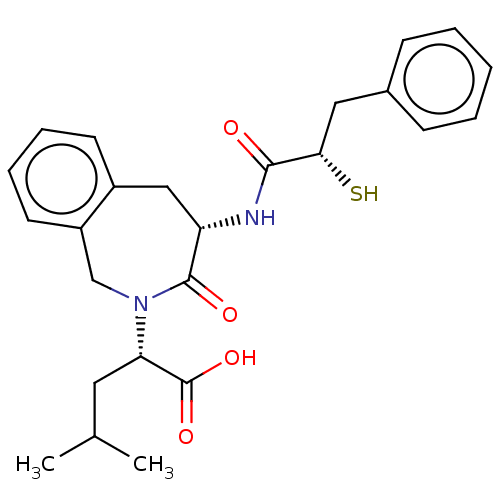
(CHEMBL5272269)Show SMILES CC(C)C[C@H](N1Cc2ccccc2C[C@H](NC(=O)[C@@H](S)Cc2ccccc2)C1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50614472
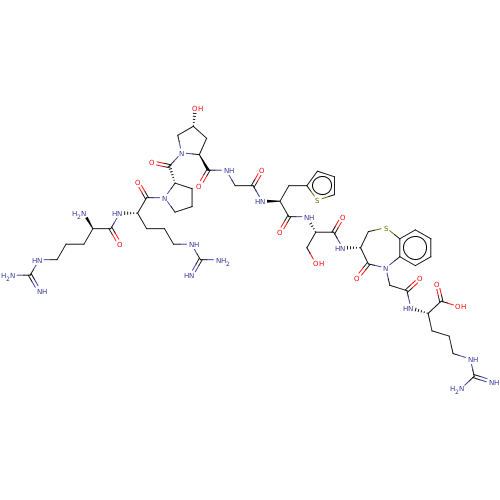
(CHEMBL5284501)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1C[C@H](O)C[C@H]1C(=O)NCC(=O)N[C@@H](Cc1cccs1)C(=O)N[C@@H](CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50403371
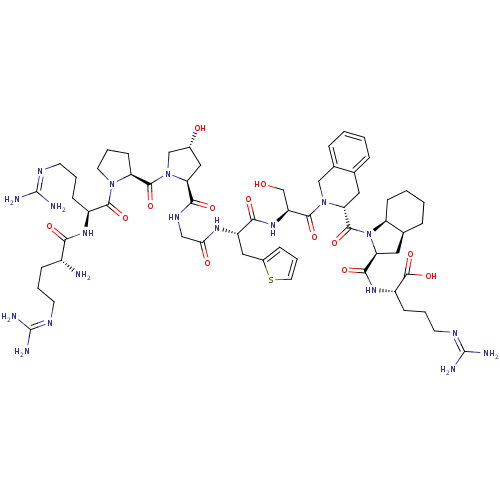
(Firazyr | HOE-140 | ICATIBANT)Show SMILES [#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1cccs1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@@H]-1-[#6](=O)-[#7]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6]-[#6@H]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C59H89N19O13S/c60-37(14-5-19-67-57(61)62)48(82)72-38(15-6-20-68-58(63)64)52(86)75-22-8-18-43(75)54(88)77-30-35(80)26-44(77)50(84)70-28-47(81)71-40(27-36-13-9-23-92-36)49(83)74-41(31-79)53(87)76-29-34-12-2-1-10-32(34)24-46(76)55(89)78-42-17-4-3-11-33(42)25-45(78)51(85)73-39(56(90)91)16-7-21-69-59(65)66/h1-2,9-10,12-13,23,33,35,37-46,79-80H,3-8,11,14-22,24-31,60H2,(H,70,84)(H,71,81)(H,72,82)(H,73,85)(H,74,83)(H,90,91)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t33-,35+,37+,38-,39-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50614491
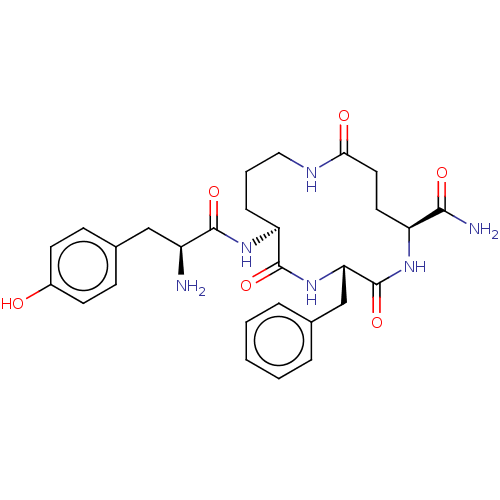
(CHEMBL5278521)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CCCNC(=O)CC[C@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50614477

(CHEMBL5267589)Show SMILES CCC[C@H](N1Cc2[nH]c3ccccc3c2C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CN)C(C)C)[C@@H](C)CC)C1=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50024102

(((S)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50614464
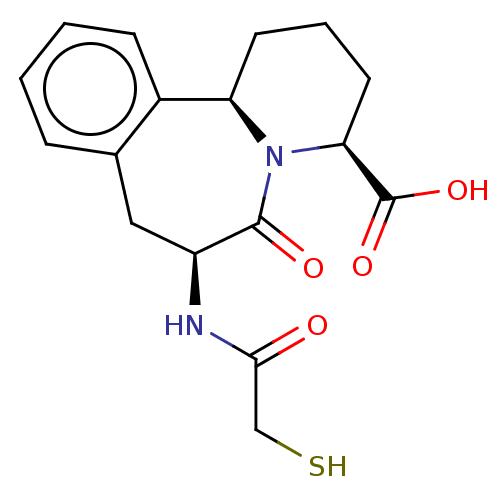
(CHEMBL5281720)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)NC(=O)CS)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50614471
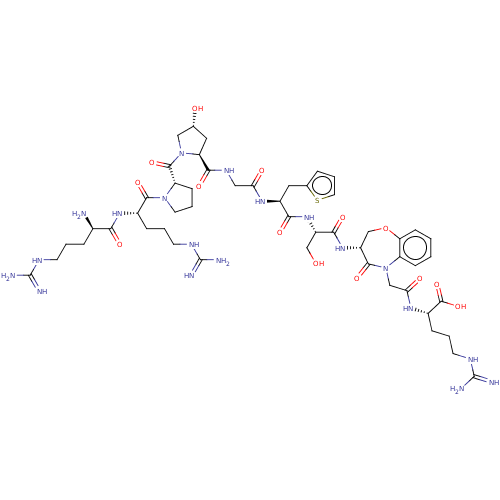
(CHEMBL5266765)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1C[C@H](O)C[C@H]1C(=O)NCC(=O)N[C@@H](Cc1cccs1)C(=O)N[C@@H](CO)C(=O)N[C@@H]1COc2ccccc2N(CC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50614491

(CHEMBL5278521)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CCCNC(=O)CC[C@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50614492
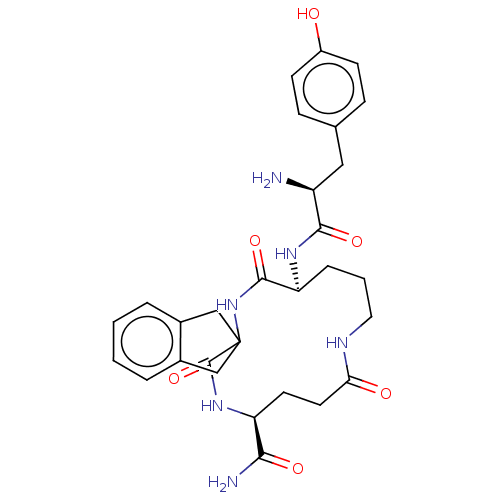
(CHEMBL5282233)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CCCNC(=O)CC[C@H](NC(=O)C2(Cc3ccccc3C2)NC1=O)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50614463

(CHEMBL5283332)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)NC(=O)[C@H](S)Cc1ccccc1)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50614493
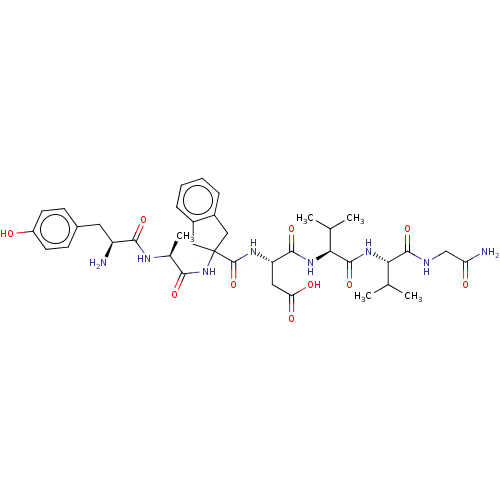
(CHEMBL5277977)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)C1(Cc2ccccc2C1)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50614464

(CHEMBL5281720)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)NC(=O)CS)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50614467
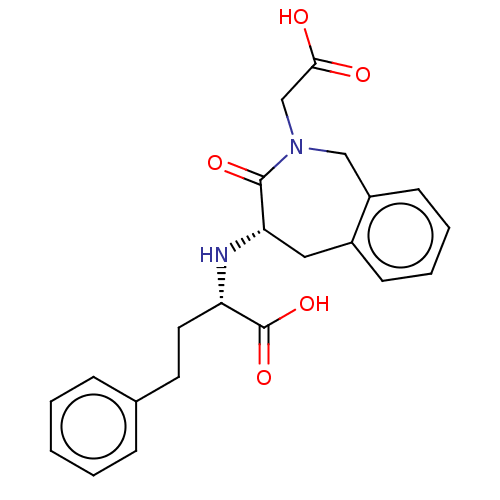
(CHEMBL5279614)Show SMILES OC(=O)CN1Cc2ccccc2C[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50614466

(CHEMBL5272537)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)N[C@@H](CCc1ccccc1)C(O)=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50614470
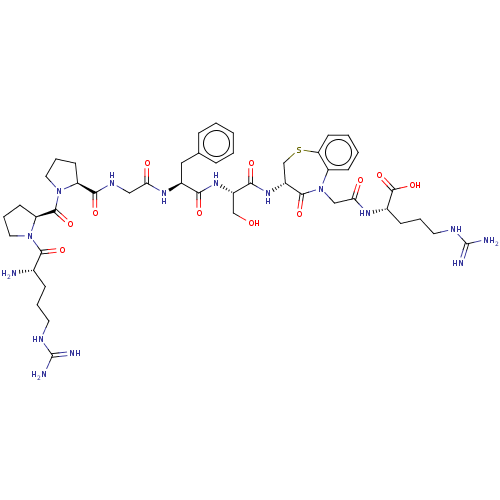
(CHEMBL5279008)Show SMILES N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H]1CSc2ccccc2N(CC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50103430

(6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28-,30+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50614487
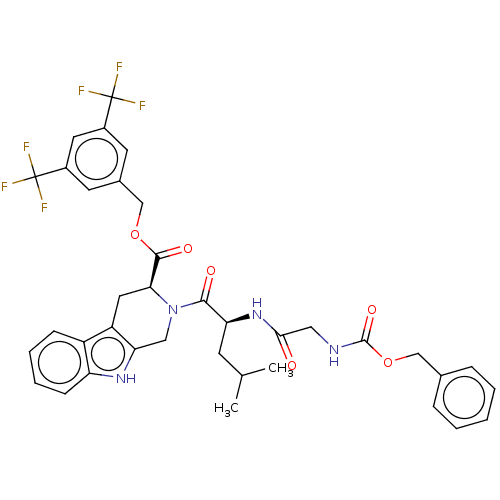
(CHEMBL5278537)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)OCc1ccccc1)C(=O)N1Cc2[nH]c3ccccc3c2C[C@H]1C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50103430

(6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50024102

(((S)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50103430

(6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28-,30+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50614489
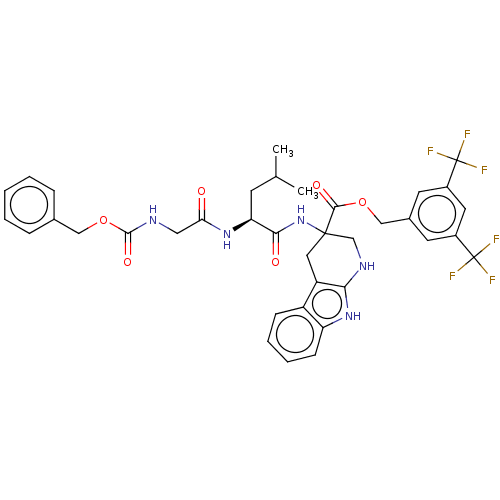
(CHEMBL5271987)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)OCc1ccccc1)C(=O)NC1(CNc2[nH]c3ccccc3c2C1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50614492

(CHEMBL5282233)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CCCNC(=O)CC[C@H](NC(=O)C2(Cc3ccccc3C2)NC1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50614465
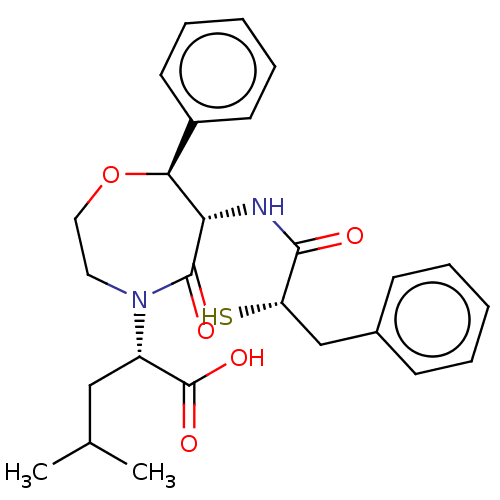
(CHEMBL5266415)Show SMILES CC(C)C[C@H](N1CCO[C@H]([C@H](NC(=O)[C@@H](S)Cc2ccccc2)C1=O)c1ccccc1)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50614486
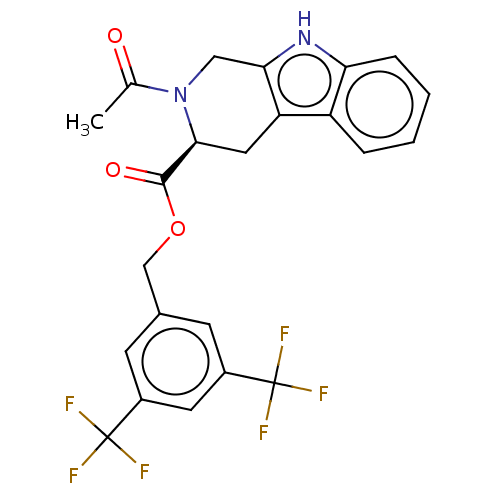
(CHEMBL5286117)Show SMILES CC(=O)N1Cc2[nH]c3ccccc3c2C[C@H]1C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50614473
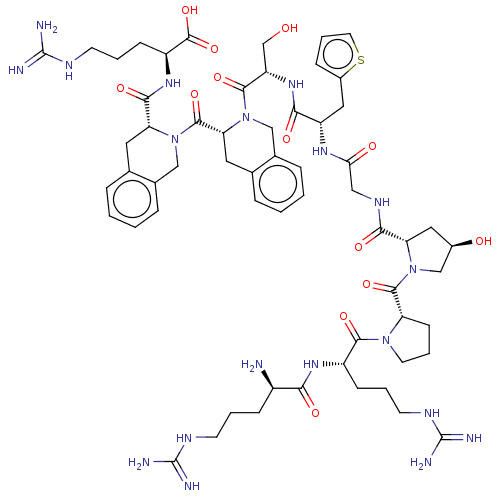
(CHEMBL5271423)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1C[C@H](O)C[C@H]1C(=O)NCC(=O)N[C@@H](Cc1cccs1)C(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 571 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50614488
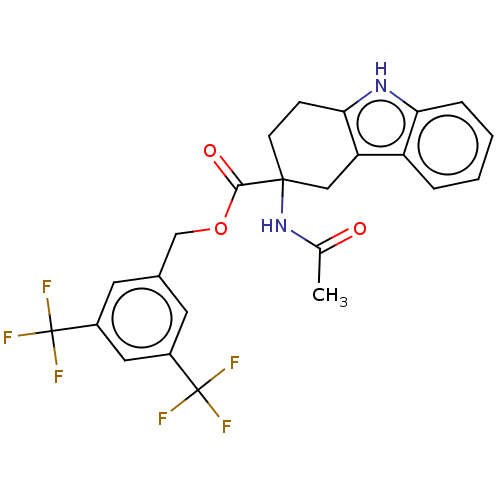
(CHEMBL5266880)Show SMILES CC(=O)NC1(CCc2[nH]c3ccccc3c2C1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 765 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50614475
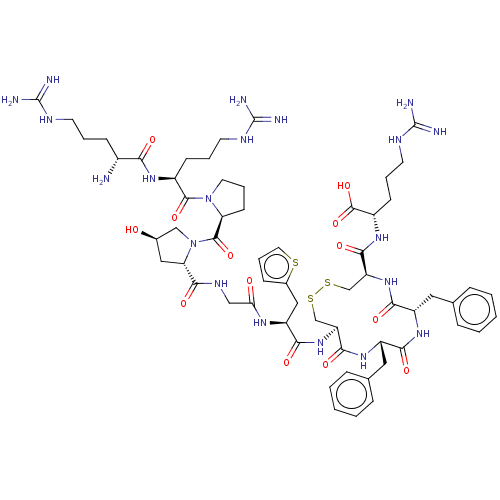
(CHEMBL5274916)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1C[C@H](O)C[C@H]1C(=O)NCC(=O)N[C@@H](Cc1cccs1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 876 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50103430

(6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28-,30+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50614465

(CHEMBL5266415)Show SMILES CC(C)C[C@H](N1CCO[C@H]([C@H](NC(=O)[C@@H](S)Cc2ccccc2)C1=O)c1ccccc1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50614481
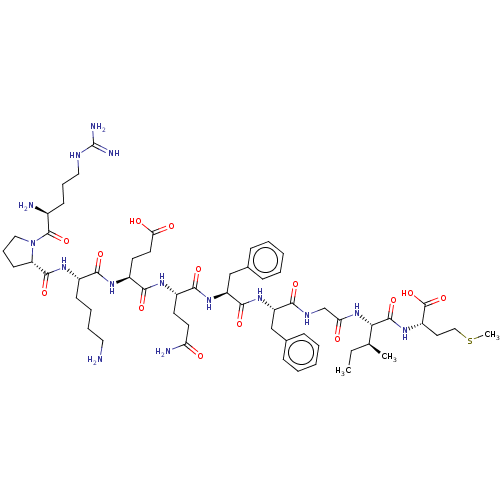
(CHEMBL5269970)Show SMILES CC[C@H](C)[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50614469
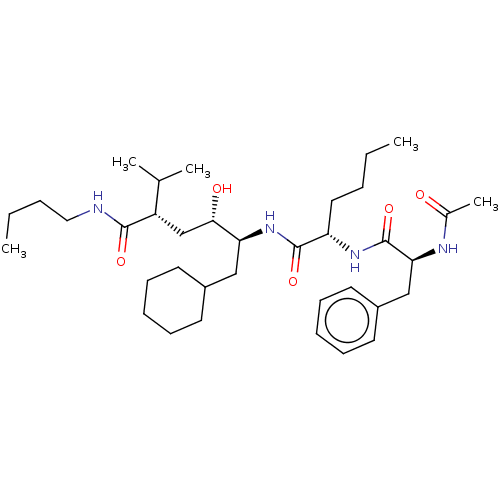
(CHEMBL5288530)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCC)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM21016
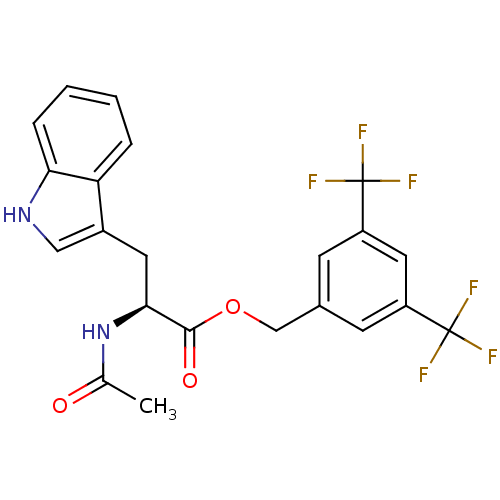
(CHEMBL22870 | L 732138 | L-732,138 | L732138 | N-a...)Show SMILES CC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C22H18F6N2O3/c1-12(31)30-19(8-14-10-29-18-5-3-2-4-17(14)18)20(32)33-11-13-6-15(21(23,24)25)9-16(7-13)22(26,27)28/h2-7,9-10,19,29H,8,11H2,1H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021127
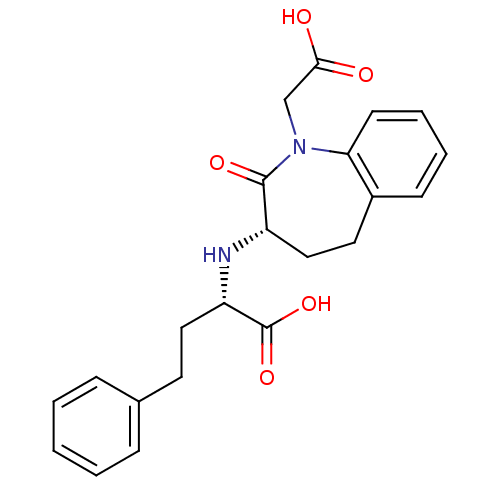
(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5/c25-20(26)14-24-19-9-5-4-8-16(19)11-13-17(21(24)27)23-18(22(28)29)12-10-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50282845
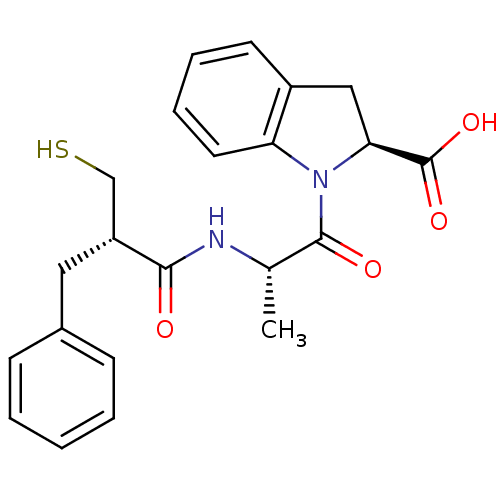
((S)-1-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-propio...)Show SMILES C[C@H](NC(=O)[C@@H](CS)Cc1ccccc1)C(=O)N1[C@@H](Cc2ccccc12)C(O)=O Show InChI InChI=1S/C22H24N2O4S/c1-14(23-20(25)17(13-29)11-15-7-3-2-4-8-15)21(26)24-18-10-6-5-9-16(18)12-19(24)22(27)28/h2-10,14,17,19,29H,11-13H2,1H3,(H,23,25)(H,27,28)/t14-,17+,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50421824
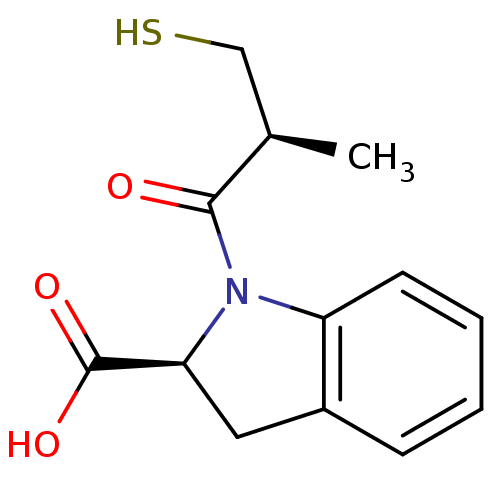
(CHEMBL75752)Show InChI InChI=1S/C13H15NO3S/c1-8(7-18)12(15)14-10-5-3-2-4-9(10)6-11(14)13(16)17/h2-5,8,11,18H,6-7H2,1H3,(H,16,17)/t8-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367258
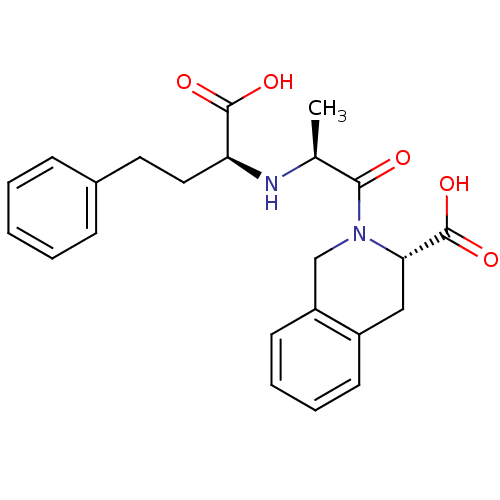
(CI-928 | QUINAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C23H26N2O5/c1-15(24-19(22(27)28)12-11-16-7-3-2-4-8-16)21(26)25-14-18-10-6-5-9-17(18)13-20(25)23(29)30/h2-10,15,19-20,24H,11-14H2,1H3,(H,27,28)(H,29,30)/t15-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50614459
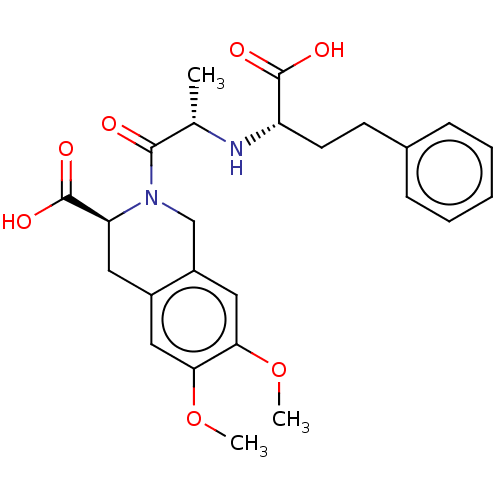
(MOEXIPRILAT | Moexipril related compound a | Moexi...)Show SMILES COc1cc2C[C@H](N(Cc2cc1OC)C(=O)[C@H](C)N[C@@H](CCc1ccccc1)C(O)=O)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50614485
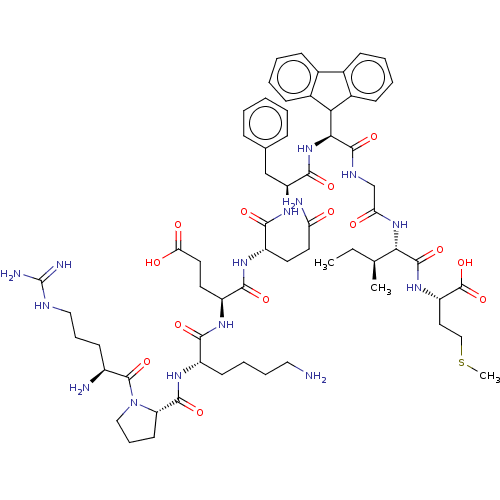
(CHEMBL5282996)Show SMILES CC[C@H](C)[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N)C1c2ccccc2-c2ccccc12)C(=O)N[C@@H](CCSC)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50614484
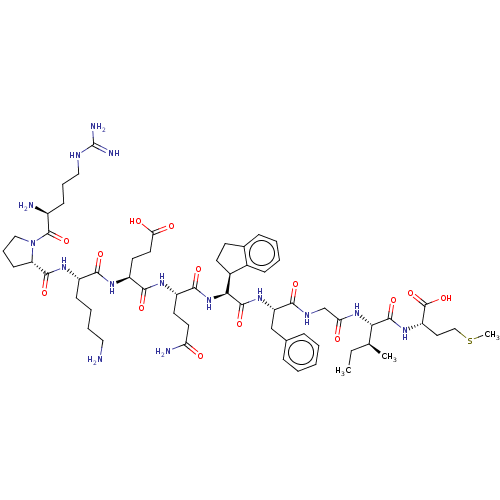
(CHEMBL5268460)Show SMILES [H][C@@]1(CCc2ccccc12)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCSC)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50493988

(CHEBI:8024 | McN-A-2833 | Perindopril | S-9490)Show SMILES [H][C@]12C[C@H](N(C(=O)[C@H](C)N[C@@H](CCC)C(=O)OCC)[C@@]1([H])CCCC2)C(O)=O Show InChI InChI=1S/C19H32N2O5/c1-4-8-14(19(25)26-5-2)20-12(3)17(22)21-15-10-7-6-9-13(15)11-16(21)18(23)24/h12-16,20H,4-11H2,1-3H3,(H,23,24)/t12-,13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data