

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM10972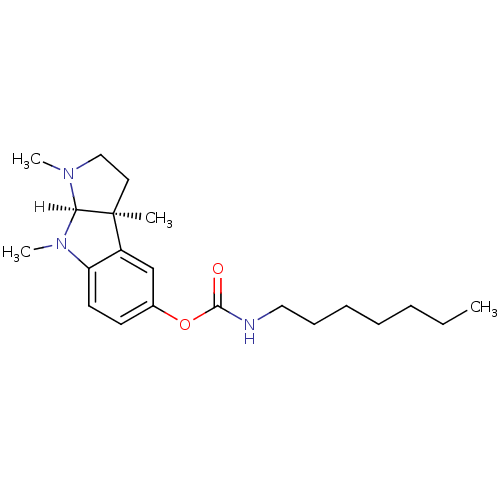 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.00029-0.001 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10972 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50290392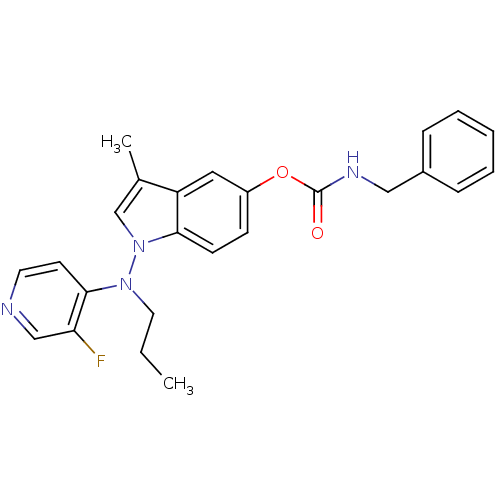 (Benzyl-carbamic acid 1-[(3-fluoro-pyridin-4-yl)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.01-0.069 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50290391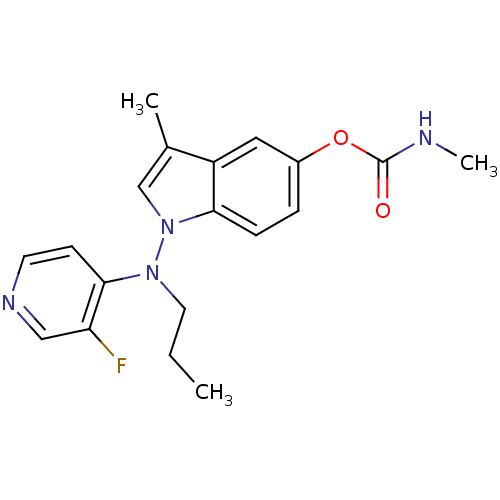 (CHEMBL42531 | Methyl-carbamic acid 1-[(3-fluoro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.023-0.041 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50290392 (Benzyl-carbamic acid 1-[(3-fluoro-pyridin-4-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50290391 (CHEMBL42531 | Methyl-carbamic acid 1-[(3-fluoro-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50290389 (CHEMBL295462 | Methyl-carbamic acid 1-(3-fluoro-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50290389 (CHEMBL295462 | Methyl-carbamic acid 1-(3-fluoro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.15-0.22 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50290390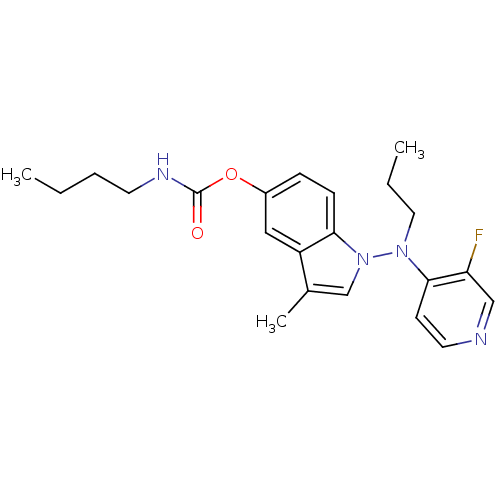 (Butyl-carbamic acid 1-[(3-fluoro-pyridin-4-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.69-1.2 | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50290390 (Butyl-carbamic acid 1-[(3-fluoro-pyridin-4-yl)-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 3.3... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50290393 (CHEMBL42667 | Heptyl-carbamic acid 1-[(3-fluoro-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 21-... | Bioorg Med Chem Lett 7: 157-162 (1997) Article DOI: 10.1016/S0960-894X(96)00592-6 BindingDB Entry DOI: 10.7270/Q2CC10P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||