

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50306339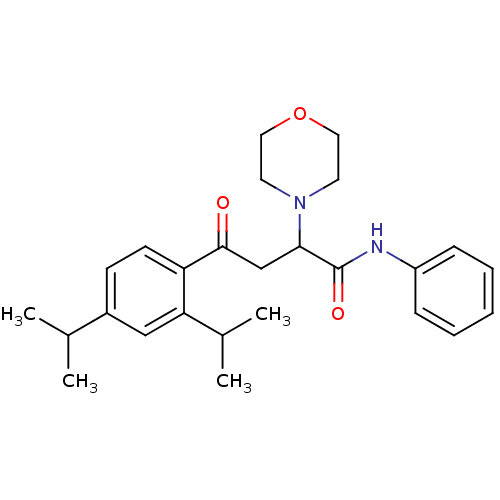 (4-(2,4-Diisopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Lineweaver-Burk plot | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306337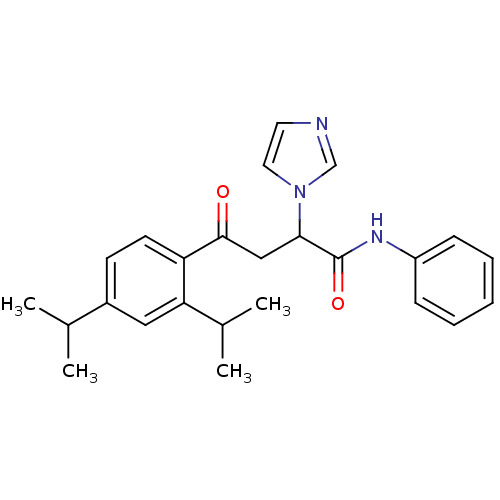 (4-(2,4-Diisopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Lineweaver-burk plot | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 43.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306337 (4-(2,4-Diisopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50306339 (4-(2,4-Diisopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306342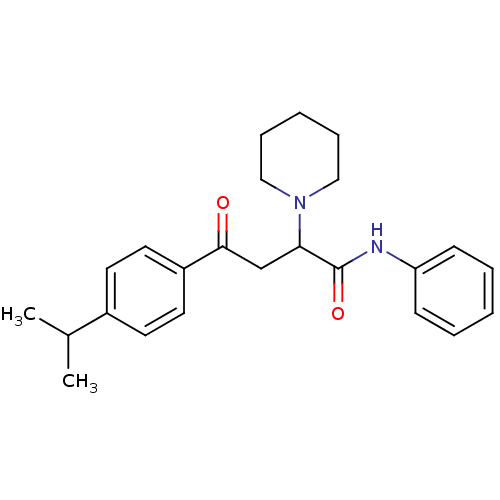 (4-(4-Isopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-pi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306352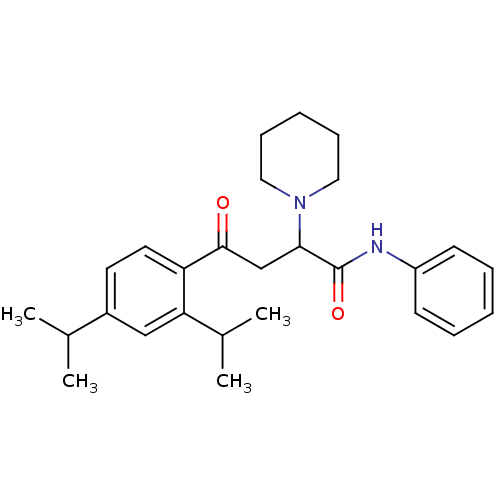 (4-(2,4-Diisopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306351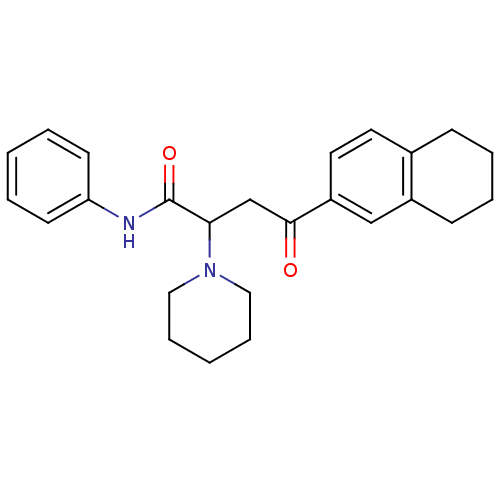 (4-(5,6,7,8-Tetrahydro-2-naphthalenyl)-4-oxo-N-phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306345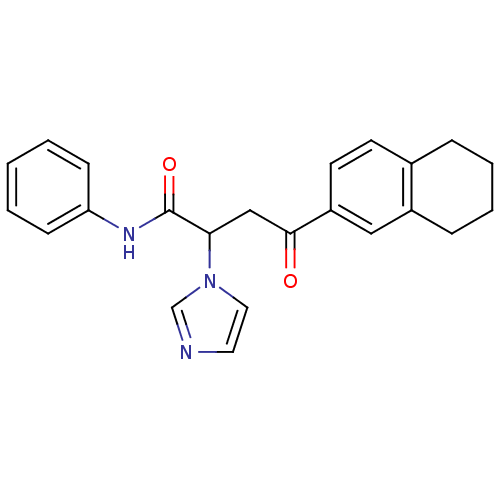 (4-(5,6,7,8-Tetrahydro-2-naphthalenyl)-4-oxo-N-phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50306342 (4-(4-Isopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-pi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306338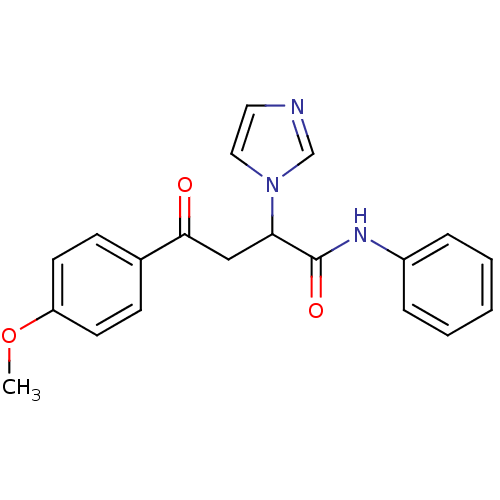 (4-(4-Metoxyphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-imida...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306346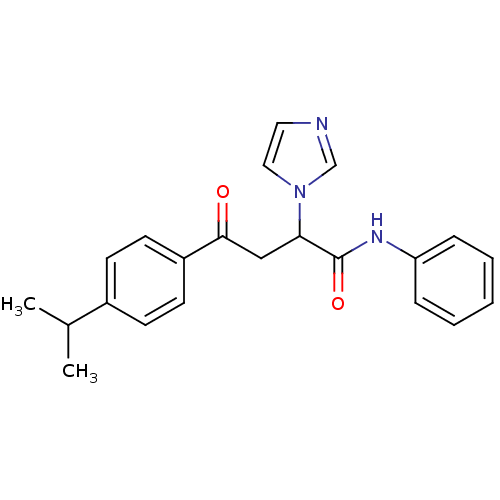 (4-(4-Isopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-im...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306339 (4-(2,4-Diisopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306336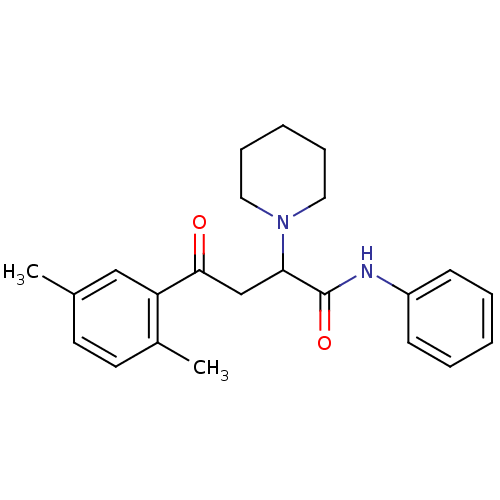 (4-(2,5-Dimethylphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306343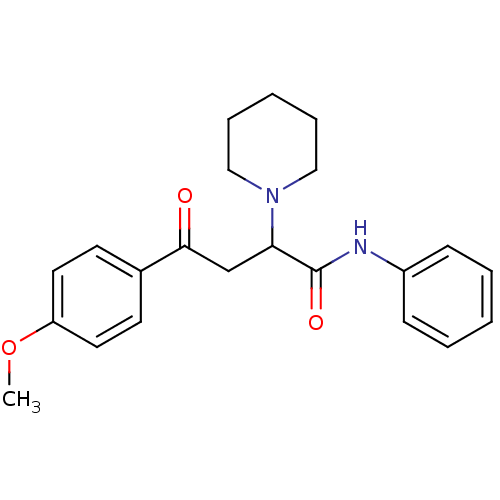 (4-(4-Metoxyphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-piper...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50306336 (4-(2,5-Dimethylphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50306337 (4-(2,4-Diisopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306347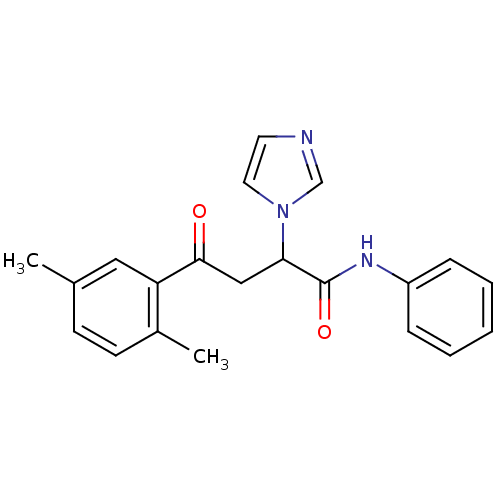 (4-(2,5-Dimethylphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-i...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306348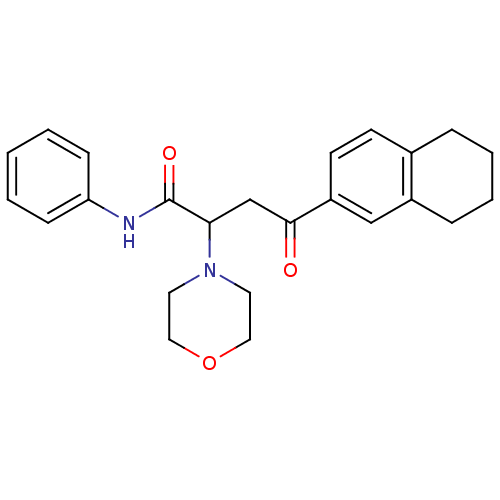 (4-(5,6,7,8-Tetrahydro-2-naphthalenyl)-4-oxo-N-phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50306338 (4-(4-Metoxyphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-imida...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50306341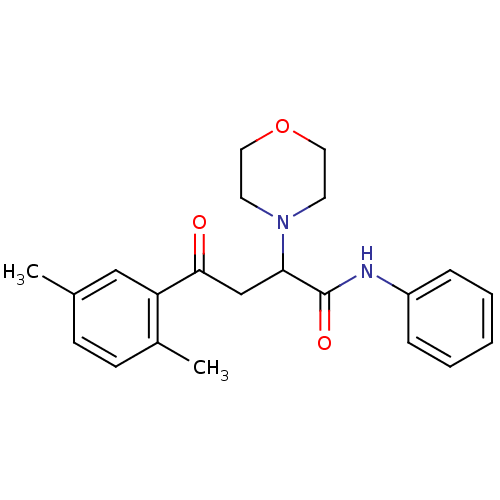 (4-(2,5-Dimethylphenyl)-4-oxo-N-phenyl-2-(R,S)-(4-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50306343 (4-(4-Metoxyphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-piper...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306344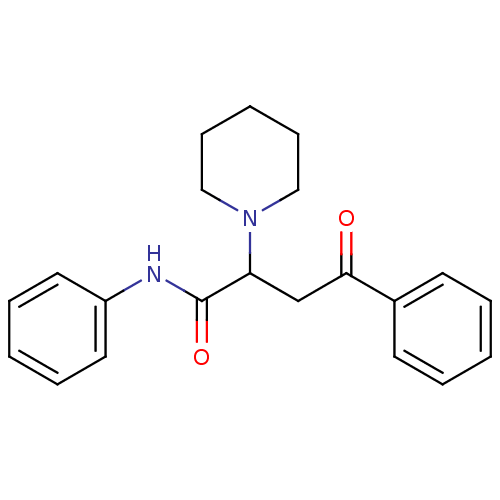 (CHEMBL610494 | Phenyl-4-oxo-N-phenyl-2-(R,S)-(1-pi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50306340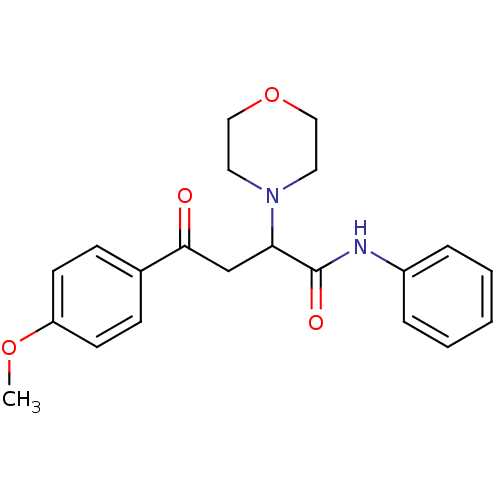 (4-(4-Metoxyphenyl)-4-oxo-N-phenyl-2-(R,S)-(4-morph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306350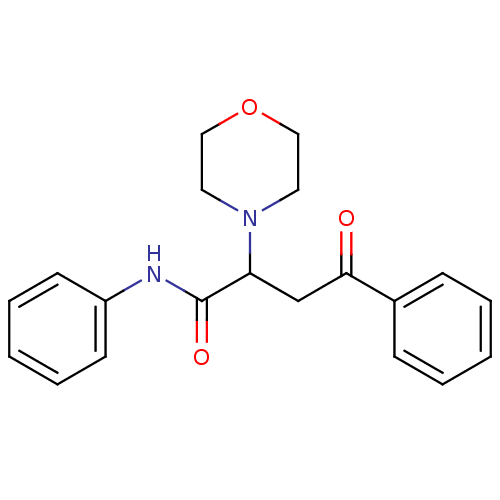 (4-Phenyl-4-oxo-N-phenyl-2-(R,S)-(4-morpholinyl)but...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306341 (4-(2,5-Dimethylphenyl)-4-oxo-N-phenyl-2-(R,S)-(4-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306349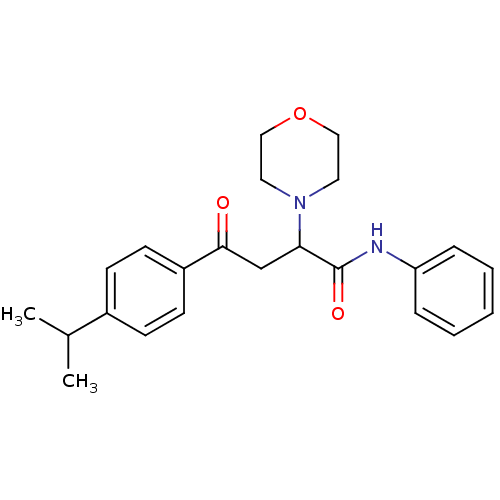 (4-(4-Isopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(4-mo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50423742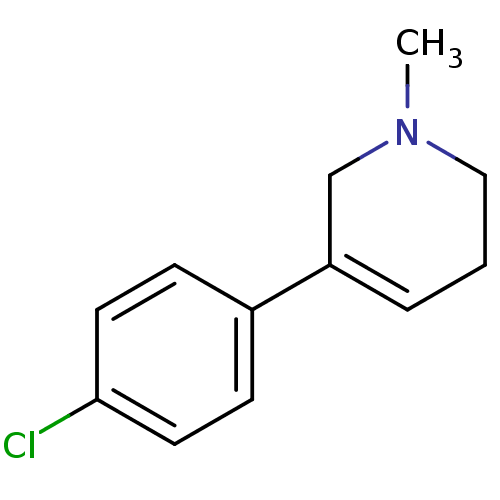 (CHEMBL602450) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50423741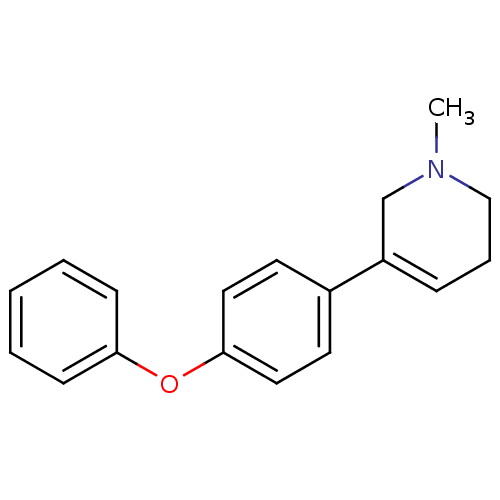 (CHEMBL589998) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50423740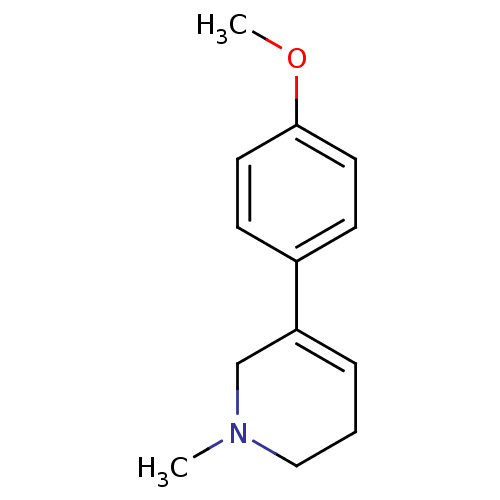 (CHEMBL602245) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306337 (4-(2,4-Diisopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50423748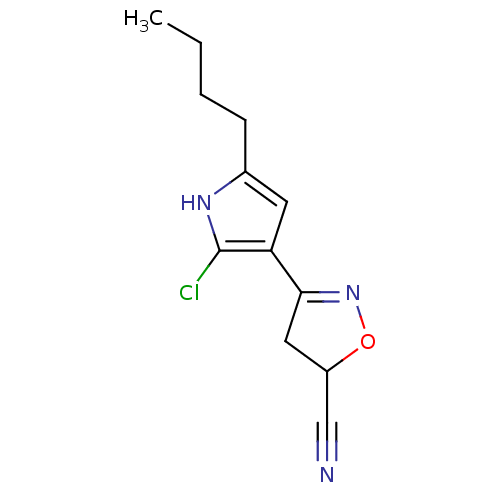 (CHEMBL598711) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306342 (4-(4-Isopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-pi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306352 (4-(2,4-Diisopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.86E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306351 (4-(5,6,7,8-Tetrahydro-2-naphthalenyl)-4-oxo-N-phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.64E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306345 (4-(5,6,7,8-Tetrahydro-2-naphthalenyl)-4-oxo-N-phen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.34E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306338 (4-(4-Metoxyphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-imida...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.85E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306346 (4-(4-Isopropylphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-im...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.22E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50423744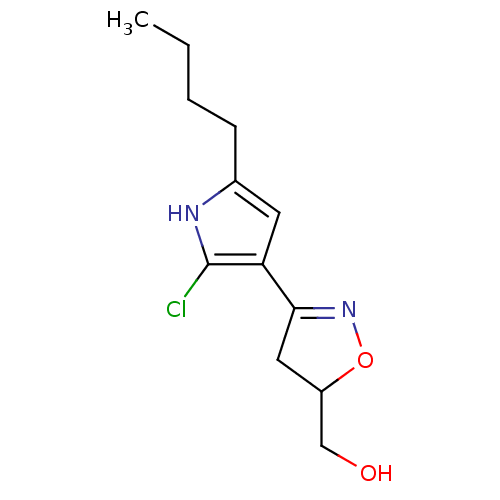 (CHEMBL592364) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50306347 (4-(2,5-Dimethylphenyl)-4-oxo-N-phenyl-2-(R,S)-(1-i...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50423746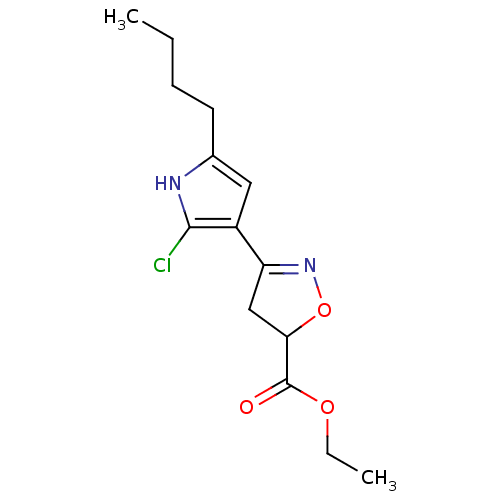 (CHEMBL597312) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.97E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50423747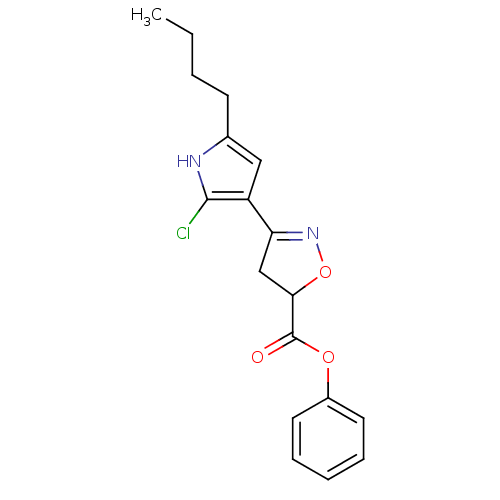 (CHEMBL598712) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.21E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50423745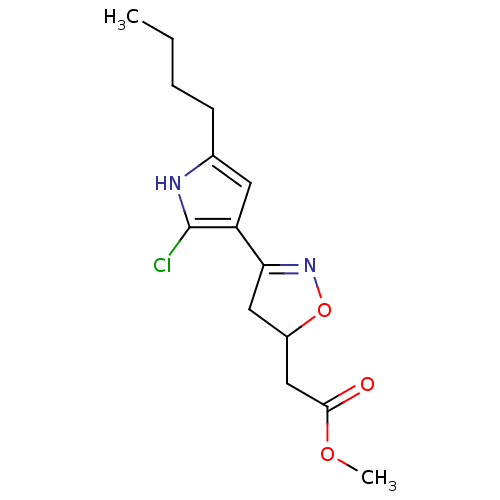 (CHEMBL599759) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50423743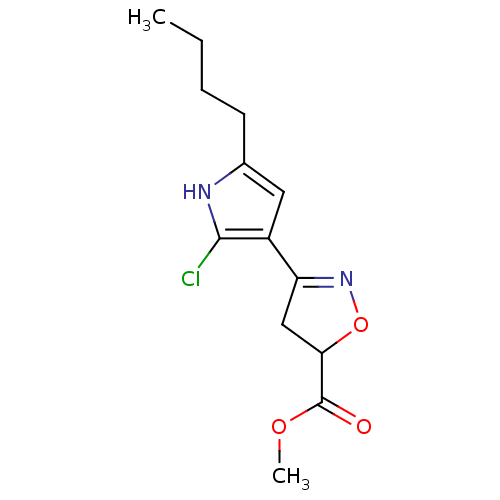 (CHEMBL598710) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.41E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem 18: 1181-93 (2010) Article DOI: 10.1016/j.bmc.2009.12.042 BindingDB Entry DOI: 10.7270/Q2ZW1M1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||