 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50031166
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50031166 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50308418

(CHEMBL605824 | N-[2-(N',N'-diisopropylamin...)Show InChI InChI=1S/C21H27N3OS/c1-15(2)23(16(3)4)14-13-22-21(25)24-17-9-5-7-11-19(17)26-20-12-8-6-10-18(20)24/h5-12,15-16H,13-14H2,1-4H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308420
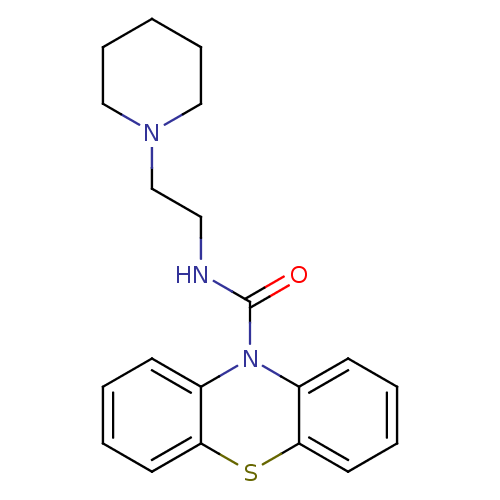
(CHEMBL589563 | N-[2-(Piperidinyl)ethyl]-10H-phenot...)Show InChI InChI=1S/C20H23N3OS/c24-20(21-12-15-22-13-6-1-7-14-22)23-16-8-2-4-10-18(16)25-19-11-5-3-9-17(19)23/h2-5,8-11H,1,6-7,12-15H2,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308409
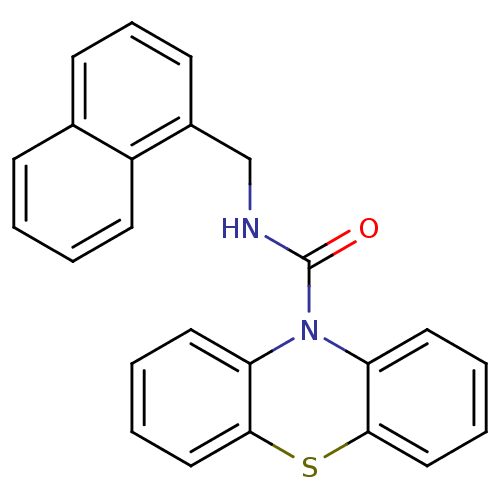
(CHEMBL592673 | N-(Naphthalen-1-ylmethyl)-1'H-pheno...)Show InChI InChI=1S/C24H18N2OS/c27-24(25-16-18-10-7-9-17-8-1-2-11-19(17)18)26-20-12-3-5-14-22(20)28-23-15-6-4-13-21(23)26/h1-15H,16H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308413
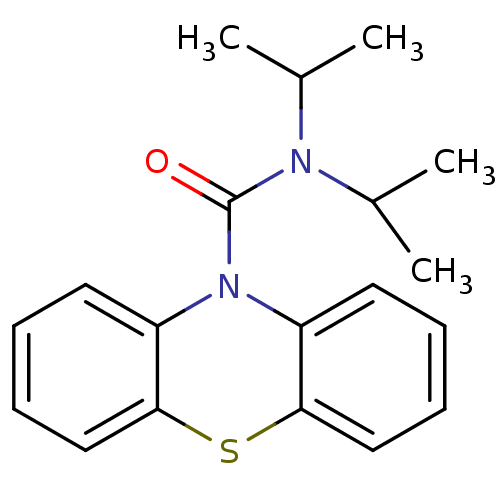
(CHEMBL588172 | N,N-Diisopropyl-1'H-phenothiazine-1...)Show InChI InChI=1S/C19H22N2OS/c1-13(2)20(14(3)4)19(22)21-15-9-5-7-11-17(15)23-18-12-8-6-10-16(18)21/h5-14H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308408
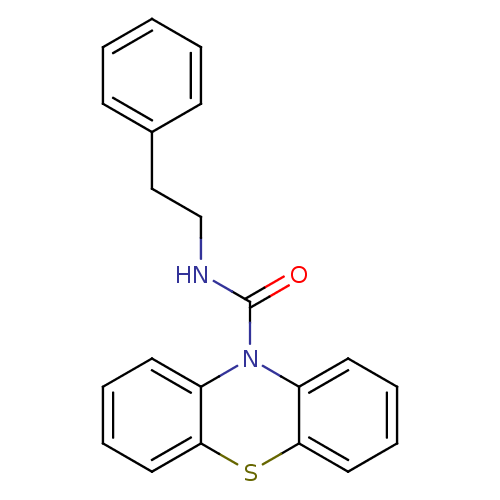
(CHEMBL602314 | N-(2-Phenylethyl)-1'H-phenothiazine...)Show InChI InChI=1S/C21H18N2OS/c24-21(22-15-14-16-8-2-1-3-9-16)23-17-10-4-6-12-19(17)25-20-13-7-5-11-18(20)23/h1-13H,14-15H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308419
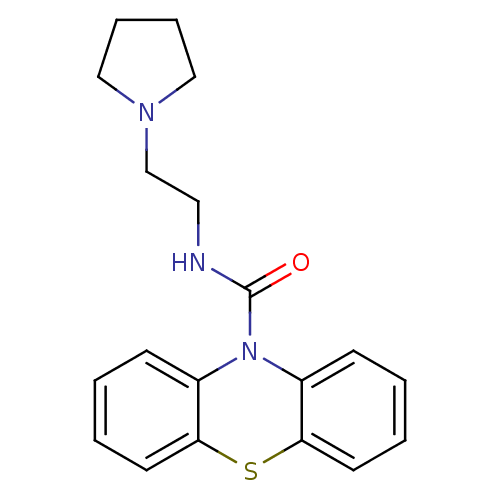
(CHEMBL589536 | N-[2-(Pyrrolidinyl)ethyl]-10H-pheno...)Show InChI InChI=1S/C19H21N3OS/c23-19(20-11-14-21-12-5-6-13-21)22-15-7-1-3-9-17(15)24-18-10-4-2-8-16(18)22/h1-4,7-10H,5-6,11-14H2,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308424
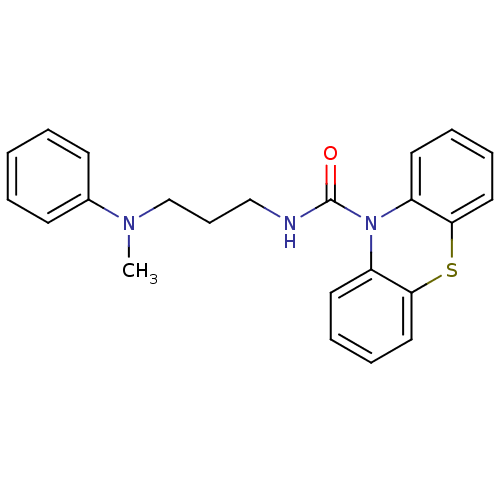
(CHEMBL589805 | N-(3-(Methyl(phenyl)amino)propyl)-1...)Show InChI InChI=1S/C23H23N3OS/c1-25(18-10-3-2-4-11-18)17-9-16-24-23(27)26-19-12-5-7-14-21(19)28-22-15-8-6-13-20(22)26/h2-8,10-15H,9,16-17H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308402
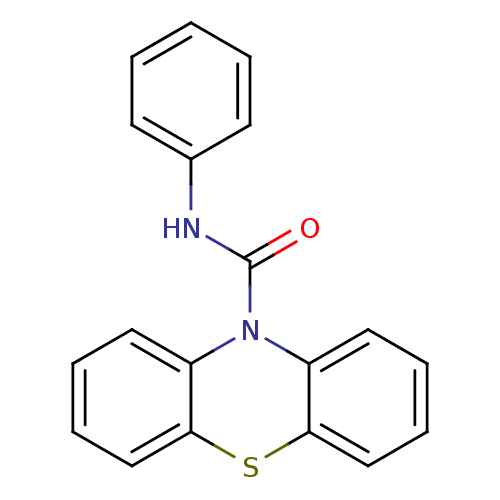
(CHEMBL590537 | N-Phenyl-1'H-phenothiazine-1'-carbo...)Show InChI InChI=1S/C19H14N2OS/c22-19(20-14-8-2-1-3-9-14)21-15-10-4-6-12-17(15)23-18-13-7-5-11-16(18)21/h1-13H,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308401
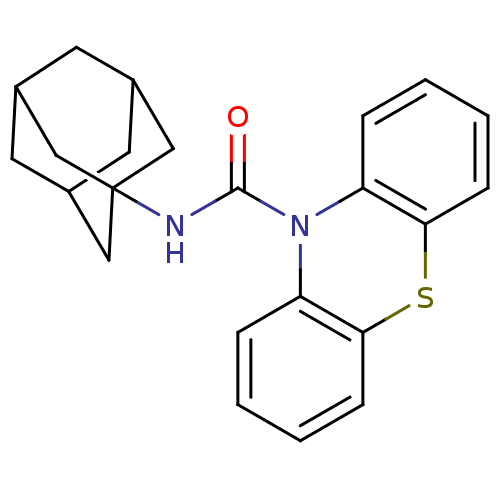
(CHEMBL591468 | N-(Adamant-1-yl)-1'H-phenothiazine-...)Show SMILES O=C(NC12CC3CC(CC(C3)C1)C2)N1c2ccccc2Sc2ccccc12 |TLB:6:7:4.5.10:11,THB:6:5:11:12.7.8,8:7:4:10.9.11,8:9:4:12.6.7| Show InChI InChI=1S/C23H24N2OS/c26-22(24-23-12-15-9-16(13-23)11-17(10-15)14-23)25-18-5-1-3-7-20(18)27-21-8-4-2-6-19(21)25/h1-8,15-17H,9-14H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308412
(CHEMBL589063 | N,N-Dipropyl-1'H-phenothiazine-1'-c...)Show InChI InChI=1S/C19H22N2OS/c1-3-13-20(14-4-2)19(22)21-15-9-5-7-11-17(15)23-18-12-8-6-10-16(18)21/h5-12H,3-4,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308417
(CHEMBL601282 | N-[2-(N',N'-Diethylamino)ethyl]-1'H...)Show InChI InChI=1S/C19H23N3OS/c1-3-21(4-2)14-13-20-19(23)22-15-9-5-7-11-17(15)24-18-12-8-6-10-16(18)22/h5-12H,3-4,13-14H2,1-2H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308414
((1'H-Phenothiazin-1'-yl)(pyrrolidin-1-yl)methanone...)Show InChI InChI=1S/C17H16N2OS/c20-17(18-11-5-6-12-18)19-13-7-1-3-9-15(13)21-16-10-4-2-8-14(16)19/h1-4,7-10H,5-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308420

(CHEMBL589563 | N-[2-(Piperidinyl)ethyl]-10H-phenot...)Show InChI InChI=1S/C20H23N3OS/c24-20(21-12-15-22-13-6-1-7-14-22)23-16-8-2-4-10-18(16)25-19-11-5-3-9-17(19)23/h2-5,8-11H,1,6-7,12-15H2,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308406
(CHEMBL600460 | N-o-Tolyl-1'H-phenothiazine-1'-carb...)Show InChI InChI=1S/C20H16N2OS/c1-14-8-2-3-9-15(14)21-20(23)22-16-10-4-6-12-18(16)24-19-13-7-5-11-17(19)22/h2-13H,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308418

(CHEMBL605824 | N-[2-(N',N'-diisopropylamin...)Show InChI InChI=1S/C21H27N3OS/c1-15(2)23(16(3)4)14-13-22-21(25)24-17-9-5-7-11-19(17)26-20-12-8-6-10-18(20)24/h5-12,15-16H,13-14H2,1-4H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308411
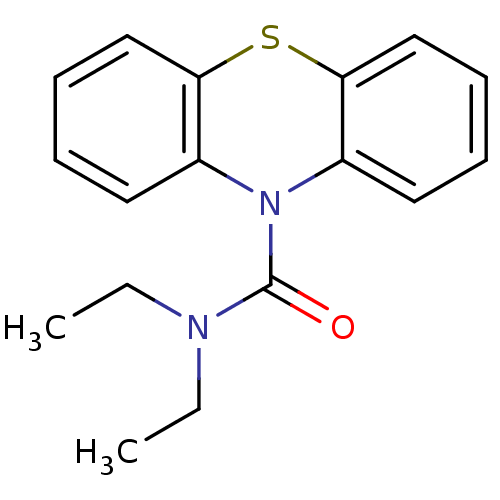
(CHEMBL589062 | N,N-Diethyl-1'H-phenothiazine-1'-ca...)Show InChI InChI=1S/C17H18N2OS/c1-3-18(4-2)17(20)19-13-9-5-7-11-15(13)21-16-12-8-6-10-14(16)19/h5-12H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308415
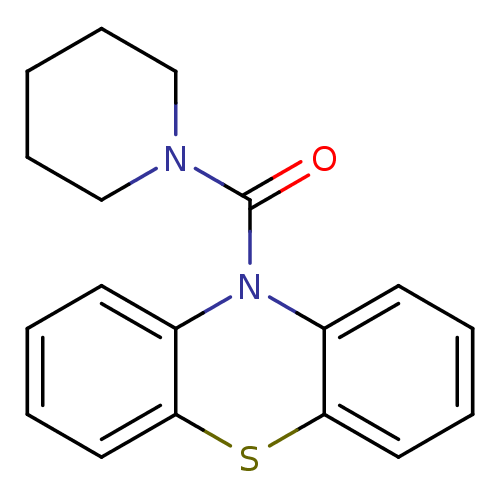
((1'H-Phenothiazin-1'-yl)(piperidin-1-yl)methanone ...)Show InChI InChI=1S/C18H18N2OS/c21-18(19-12-6-1-7-13-19)20-14-8-2-4-10-16(14)22-17-11-5-3-9-15(17)20/h2-5,8-11H,1,6-7,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308432

(CHEMBL592432 | N-Neopentyl-1'H-phenothiazine-1'-ca...)Show InChI InChI=1S/C18H20N2OS/c1-18(2,3)12-19-17(21)20-13-8-4-6-10-15(13)22-16-11-7-5-9-14(16)20/h4-11H,12H2,1-3H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308400

(CHEMBL591234 | N-Cyclohexyl-1'H-phenothiazine-1'-c...)Show InChI InChI=1S/C19H20N2OS/c22-19(20-14-8-2-1-3-9-14)21-15-10-4-6-12-17(15)23-18-13-7-5-11-16(18)21/h4-7,10-14H,1-3,8-9H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308399

(CHEMBL601297 | N-Cyclopentyl-1'H-phenothiazine-1'-...)Show InChI InChI=1S/C18H18N2OS/c21-18(19-13-7-1-2-8-13)20-14-9-3-5-11-16(14)22-17-12-6-4-10-15(17)20/h3-6,9-13H,1-2,7-8H2,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308429
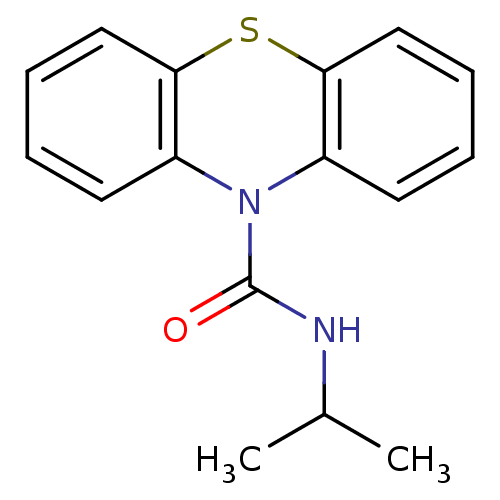
(CHEMBL592185 | N-Isopropyl-1'H-phenothiazine-1'-ca...)Show InChI InChI=1S/C16H16N2OS/c1-11(2)17-16(19)18-12-7-3-5-9-14(12)20-15-10-6-4-8-13(15)18/h3-11H,1-2H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308407
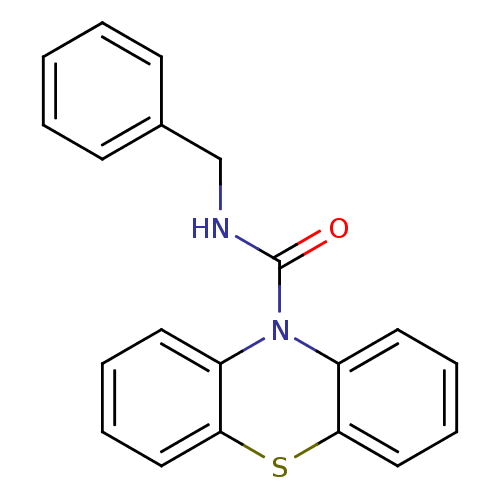
(CHEMBL589078 | N-Benzyl-1'H-phenothiazine-1'-carbo...)Show InChI InChI=1S/C20H16N2OS/c23-20(21-14-15-8-2-1-3-9-15)22-16-10-4-6-12-18(16)24-19-13-7-5-11-17(19)22/h1-13H,14H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308405
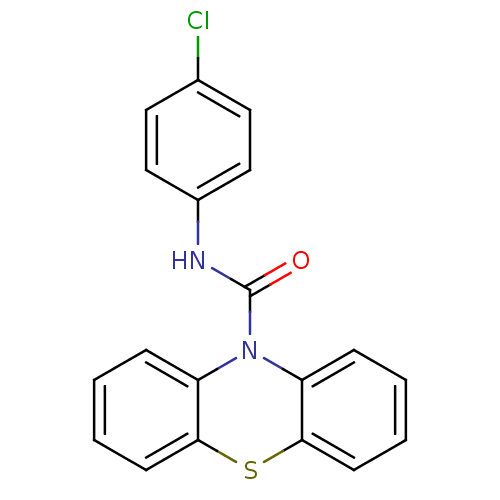
(CHEMBL590779 | N-(4-Chlorophenyl)-1'H-phenothiazin...)Show InChI InChI=1S/C19H13ClN2OS/c20-13-9-11-14(12-10-13)21-19(23)22-15-5-1-3-7-17(15)24-18-8-4-2-6-16(18)22/h1-12H,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308421
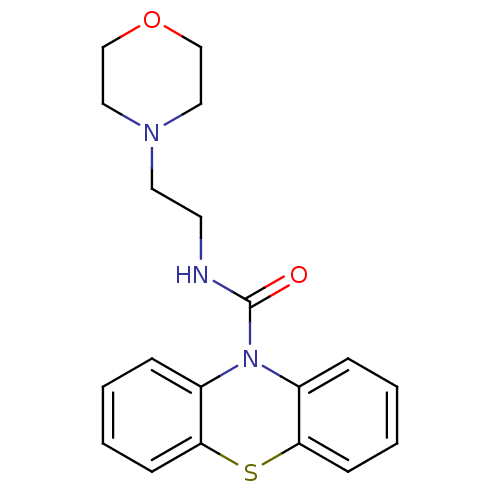
(CHEMBL589564 | N-(2-Morpholinoethyl)-10H-phenothia...)Show InChI InChI=1S/C19H21N3O2S/c23-19(20-9-10-21-11-13-24-14-12-21)22-15-5-1-3-7-17(15)25-18-8-4-2-6-16(18)22/h1-8H,9-14H2,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308416
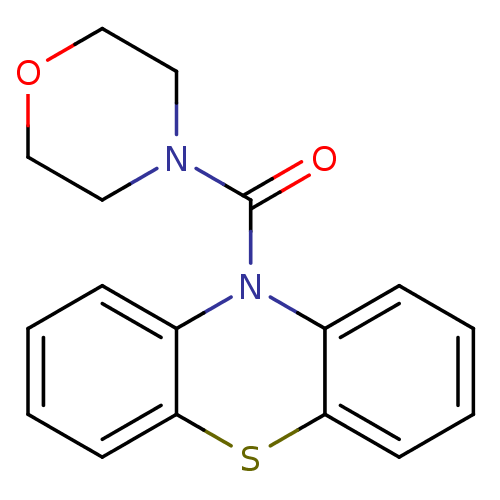
(CHEMBL590511 | Morpholino(1'H-phenothiazin-1'-yl)m...)Show InChI InChI=1S/C17H16N2O2S/c20-17(18-9-11-21-12-10-18)19-13-5-1-3-7-15(13)22-16-8-4-2-6-14(16)19/h1-8H,9-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308422

(CHEMBL589565 | N-(3-Morpholinopropyl)-10H-phenothi...)Show InChI InChI=1S/C20H23N3O2S/c24-20(21-10-5-11-22-12-14-25-15-13-22)23-16-6-1-3-8-18(16)26-19-9-4-2-7-17(19)23/h1-4,6-9H,5,10-15H2,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308410

(CHEMBL592674 | N,N-Dimethyl-1'H-phenothiazine-1'-c...)Show InChI InChI=1S/C15H14N2OS/c1-16(2)15(18)17-11-7-3-5-9-13(11)19-14-10-6-4-8-12(14)17/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308426
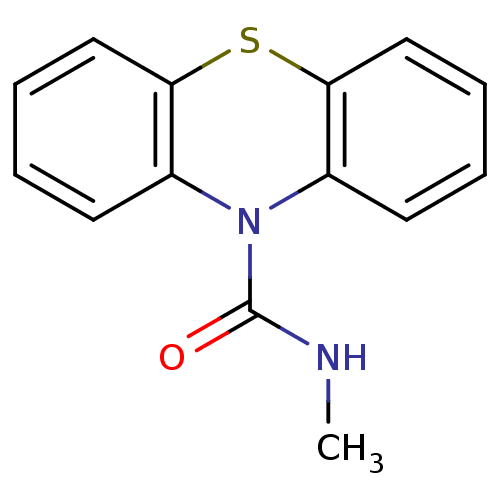
(CHEMBL606338 | N-Methyl-1'H-phenothiazine-1'-carbo...)Show InChI InChI=1S/C14H12N2OS/c1-15-14(17)16-10-6-2-4-8-12(10)18-13-9-5-3-7-11(13)16/h2-9H,1H3,(H,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308403
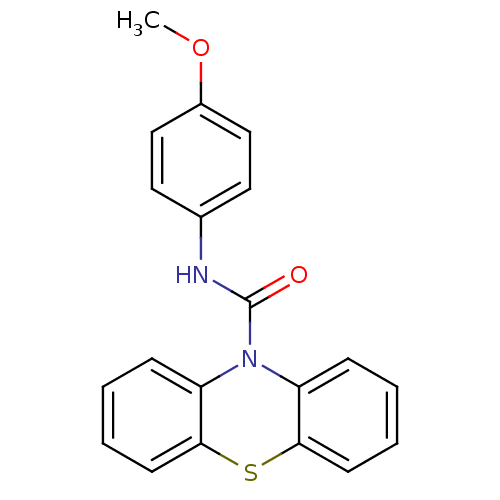
(CHEMBL601920 | N-(4-Methoxyphenyl)-1'H-phenothiazi...)Show InChI InChI=1S/C20H16N2O2S/c1-24-15-12-10-14(11-13-15)21-20(23)22-16-6-2-4-8-18(16)25-19-9-5-3-7-17(19)22/h2-13H,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308398

(CHEMBL591233 | N-Cyclobutyl-1'H-phenothiazine-1'-c...)Show InChI InChI=1S/C17H16N2OS/c20-17(18-12-6-5-7-12)19-13-8-1-3-10-15(13)21-16-11-4-2-9-14(16)19/h1-4,8-12H,5-7H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308431

(CHEMBL592431 | N-tert-Butyl-1'H-phenothiazine-1'-c...)Show InChI InChI=1S/C17H18N2OS/c1-17(2,3)18-16(20)19-12-8-4-6-10-14(12)21-15-11-7-5-9-13(15)19/h4-11H,1-3H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308404

(CHEMBL590538 | N-p-Tolyl-1'H-phenothiazine-1'-carb...)Show InChI InChI=1S/C20H16N2OS/c1-14-10-12-15(13-11-14)21-20(23)22-16-6-2-4-8-18(16)24-19-9-5-3-7-17(19)22/h2-13H,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308417

(CHEMBL601282 | N-[2-(N',N'-Diethylamino)ethyl]-1'H...)Show InChI InChI=1S/C19H23N3OS/c1-3-21(4-2)14-13-20-19(23)22-15-9-5-7-11-17(15)24-18-12-8-6-10-16(18)22/h5-12H,3-4,13-14H2,1-2H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308423

((4-Methylpiperazin-1-yl)(10H-phenothiazin-10-yl)me...)Show InChI InChI=1S/C18H19N3OS/c1-19-10-12-20(13-11-19)18(22)21-14-6-2-4-8-16(14)23-17-9-5-3-7-15(17)21/h2-9H,10-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308426

(CHEMBL606338 | N-Methyl-1'H-phenothiazine-1'-carbo...)Show InChI InChI=1S/C14H12N2OS/c1-15-14(17)16-10-6-2-4-8-12(10)18-13-9-5-3-7-11(13)16/h2-9H,1H3,(H,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308428

(CHEMBL589789 | N-Propyl-1'H-phenothiazine-1'-carbo...)Show InChI InChI=1S/C16H16N2OS/c1-2-11-17-16(19)18-12-7-3-5-9-14(12)20-15-10-6-4-8-13(15)18/h3-10H,2,11H2,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308430
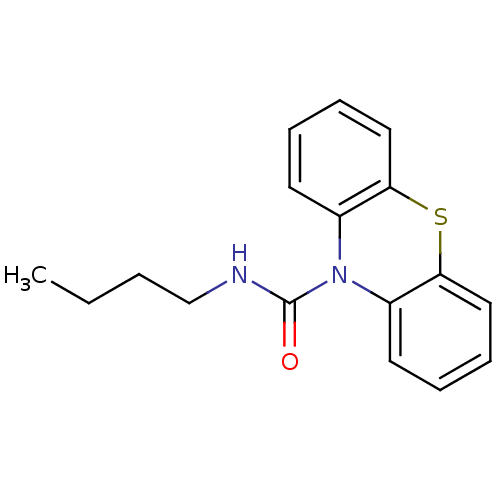
(CHEMBL592430 | N-Butyl-1'H-phenothiazine-1'-carbox...)Show InChI InChI=1S/C17H18N2OS/c1-2-3-12-18-17(20)19-13-8-4-6-10-15(13)21-16-11-7-5-9-14(16)19/h4-11H,2-3,12H2,1H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308433

(CHEMBL604046 | N-(2-Methoxyethyl)-1'H-phenothiazin...)Show InChI InChI=1S/C16H16N2O2S/c1-20-11-10-17-16(19)18-12-6-2-4-8-14(12)21-15-9-5-3-7-13(15)18/h2-9H,10-11H2,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308419

(CHEMBL589536 | N-[2-(Pyrrolidinyl)ethyl]-10H-pheno...)Show InChI InChI=1S/C19H21N3OS/c23-19(20-11-14-21-12-5-6-13-21)22-15-7-1-3-9-17(15)24-18-10-4-2-8-16(18)22/h1-4,7-10H,5-6,11-14H2,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308427
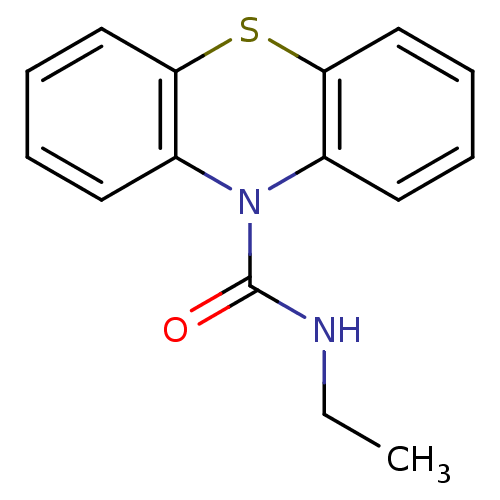
(CHEMBL600230 | N-Ethyl-1'H-phenothiazine-1'-carbox...)Show InChI InChI=1S/C15H14N2OS/c1-2-16-15(18)17-11-7-3-5-9-13(11)19-14-10-6-4-8-12(14)17/h3-10H,2H2,1H3,(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308434
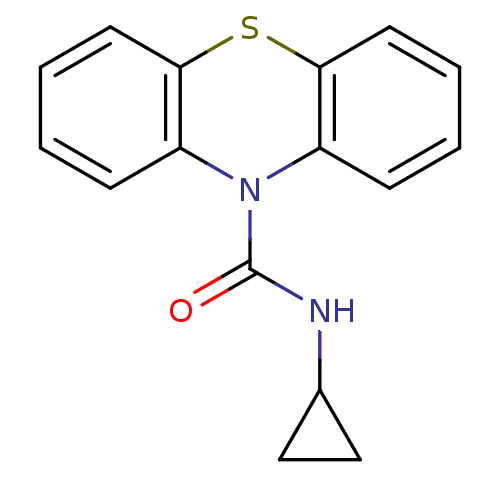
(CHEMBL591232 | N-Cyclopropyl-1'H-phenothiazine-1'-...)Show InChI InChI=1S/C16H14N2OS/c19-16(17-11-9-10-11)18-12-5-1-3-7-14(12)20-15-8-4-2-6-13(15)18/h1-8,11H,9-10H2,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308425
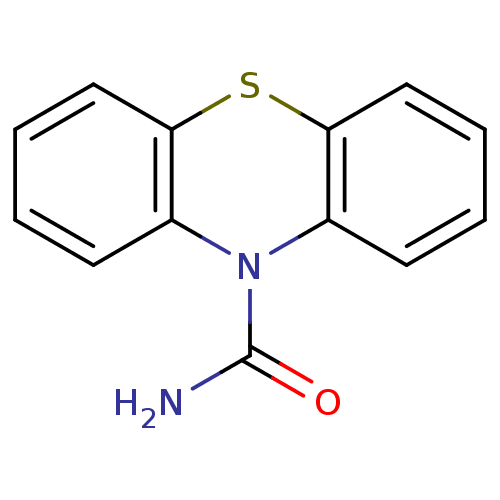
(CHEMBL590298 | Phenothiazine-1'-carboxamide)Show InChI InChI=1S/C13H10N2OS/c14-13(16)15-9-5-1-3-7-11(9)17-12-8-4-2-6-10(12)15/h1-8H,(H2,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308425

(CHEMBL590298 | Phenothiazine-1'-carboxamide)Show InChI InChI=1S/C13H10N2OS/c14-13(16)15-9-5-1-3-7-11(9)17-12-8-4-2-6-10(12)15/h1-8H,(H2,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308410

(CHEMBL592674 | N,N-Dimethyl-1'H-phenothiazine-1'-c...)Show InChI InChI=1S/C15H14N2OS/c1-16(2)15(18)17-11-7-3-5-9-13(11)19-14-10-6-4-8-12(14)17/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308429

(CHEMBL592185 | N-Isopropyl-1'H-phenothiazine-1'-ca...)Show InChI InChI=1S/C16H16N2OS/c1-11(2)17-16(19)18-12-7-3-5-9-14(12)20-15-10-6-4-8-13(15)18/h3-11H,1-2H3,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308427

(CHEMBL600230 | N-Ethyl-1'H-phenothiazine-1'-carbox...)Show InChI InChI=1S/C15H14N2OS/c1-2-16-15(18)17-11-7-3-5-9-13(11)19-14-10-6-4-8-12(14)17/h3-10H,2H2,1H3,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by modified Ellman's spectrophotometric method |
Bioorg Med Chem 18: 2232-44 (2010)
Article DOI: 10.1016/j.bmc.2010.01.066
BindingDB Entry DOI: 10.7270/Q21C1X08 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data