 Found 28 hits Enz. Inhib. hit(s) with all data for entry = 50033041
Found 28 hits Enz. Inhib. hit(s) with all data for entry = 50033041 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338666

(5,6,7-trihydroxy-2-methyl-4H-chromen-4-one | CHEMB...)Show InChI InChI=1S/C10H8O5/c1-4-2-5(11)8-7(15-4)3-6(12)9(13)10(8)14/h2-3,12-14H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338658
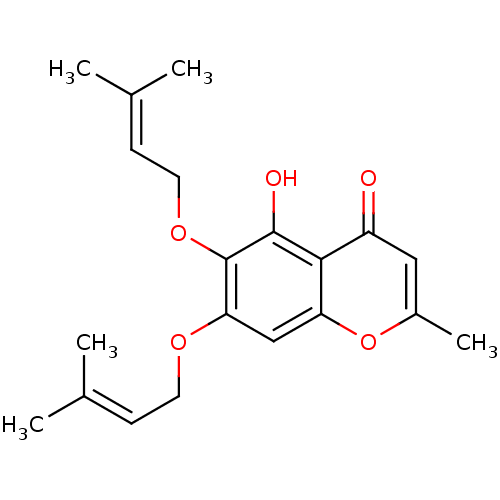
(5-hydroxy-2-methyl-6,7-bis[(3-methylbut-2-en-1-yl)...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8])c1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C20H24O5/c1-12(2)6-8-23-17-11-16-18(15(21)10-14(5)25-16)19(22)20(17)24-9-7-13(3)4/h6-7,10-11,22H,8-9H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338657
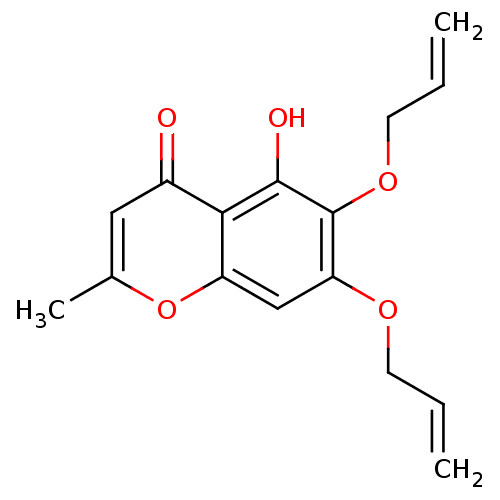
(5-hydroxy-2-methyl-6,7-bis(prop-2-en-1-yloxy)-4H-c...)Show InChI InChI=1S/C16H16O5/c1-4-6-19-13-9-12-14(11(17)8-10(3)21-12)15(18)16(13)20-7-5-2/h4-5,8-9,18H,1-2,6-7H2,3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338666

(5,6,7-trihydroxy-2-methyl-4H-chromen-4-one | CHEMB...)Show InChI InChI=1S/C10H8O5/c1-4-2-5(11)8-7(15-4)3-6(12)9(13)10(8)14/h2-3,12-14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338665
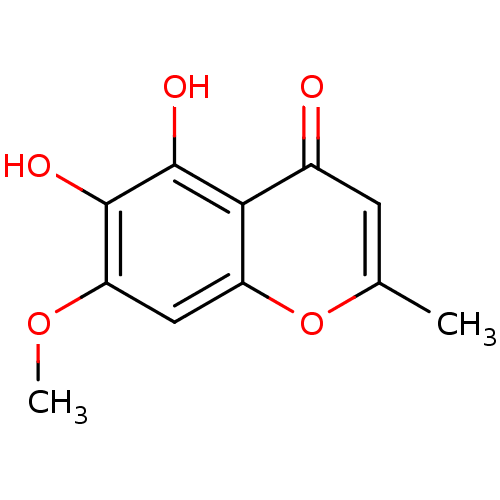
(5,6-dihydroxy-7-methoxy-2-methyl-4H-chromen-4-one ...)Show InChI InChI=1S/C11H10O5/c1-5-3-6(12)9-7(16-5)4-8(15-2)10(13)11(9)14/h3-4,13-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338668
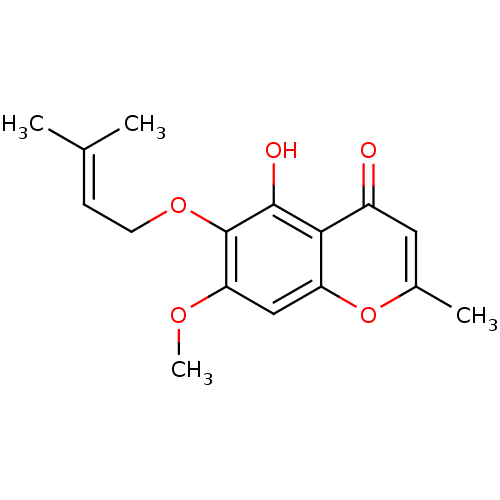
(5-hydroxy-7-methoxy-2-methyl-6-(3-methylbut-2-enyl...)Show SMILES [#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8])c1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C16H18O5/c1-9(2)5-6-20-16-13(19-4)8-12-14(15(16)18)11(17)7-10(3)21-12/h5,7-8,18H,6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338658

(5-hydroxy-2-methyl-6,7-bis[(3-methylbut-2-en-1-yl)...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8])c1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C20H24O5/c1-12(2)6-8-23-17-11-16-18(15(21)10-14(5)25-16)19(22)20(17)24-9-7-13(3)4/h6-7,10-11,22H,8-9H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338664
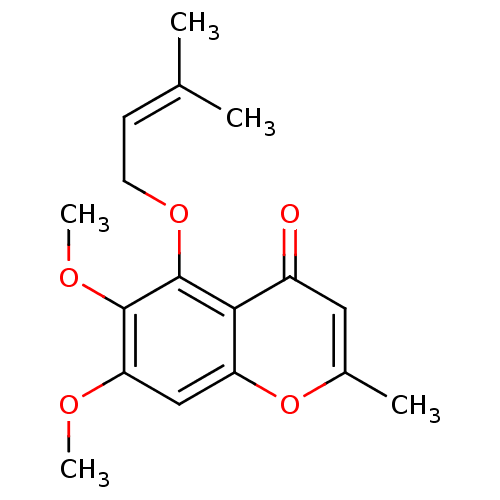
(6,7-dimethoxy-2-methyl-5-[(3-methylbut-2-en-1-yl)o...)Show SMILES [#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8]-[#6] Show InChI InChI=1S/C17H20O5/c1-10(2)6-7-21-17-15-12(18)8-11(3)22-13(15)9-14(19-4)16(17)20-5/h6,8-9H,7H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338662
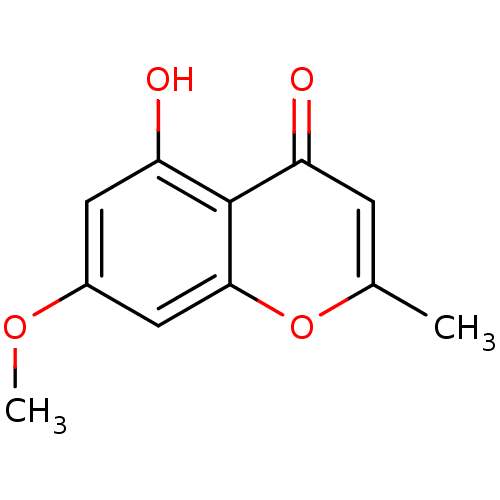
(5-hydroxy-7-methoxy-2-methyl-4H-chromen-4-one | CH...)Show InChI InChI=1S/C11H10O4/c1-6-3-8(12)11-9(13)4-7(14-2)5-10(11)15-6/h3-5,13H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338667
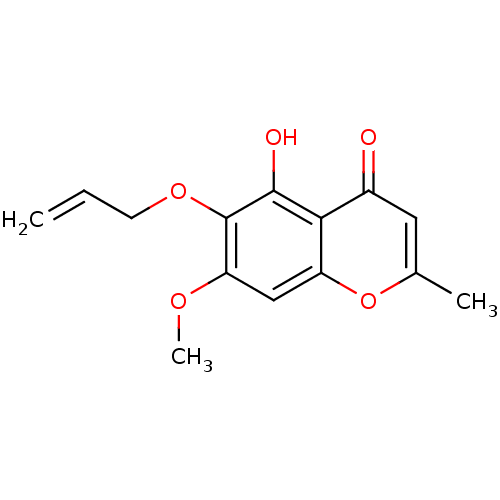
(5-hydroxy-7-methoxy-2-methyl-6-(prop-2-en-1-yloxy)...)Show InChI InChI=1S/C14H14O5/c1-4-5-18-14-11(17-3)7-10-12(13(14)16)9(15)6-8(2)19-10/h4,6-7,16H,1,5H2,2-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338668

(5-hydroxy-7-methoxy-2-methyl-6-(3-methylbut-2-enyl...)Show SMILES [#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8])c1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C16H18O5/c1-9(2)5-6-20-16-13(19-4)8-12-14(15(16)18)11(17)7-10(3)21-12/h5,7-8,18H,6H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338661
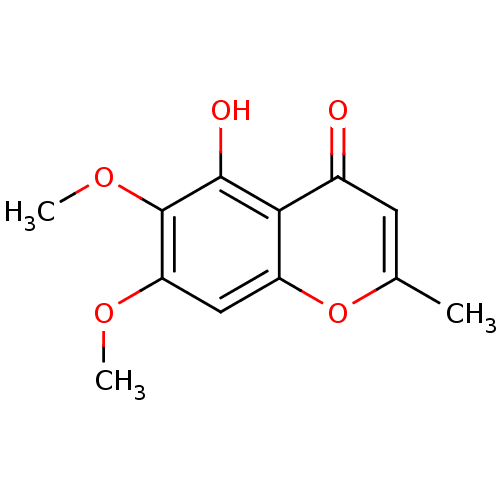
(CHEMBL1684136 | Stellatin)Show InChI InChI=1S/C12H12O5/c1-6-4-7(13)10-8(17-6)5-9(15-2)12(16-3)11(10)14/h4-5,14H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338665

(5,6-dihydroxy-7-methoxy-2-methyl-4H-chromen-4-one ...)Show InChI InChI=1S/C11H10O5/c1-5-3-6(12)9-7(16-5)4-8(15-2)10(13)11(9)14/h3-4,13-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338662

(5-hydroxy-7-methoxy-2-methyl-4H-chromen-4-one | CH...)Show InChI InChI=1S/C11H10O4/c1-6-3-8(12)11-9(13)4-7(14-2)5-10(11)15-6/h3-5,13H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338663
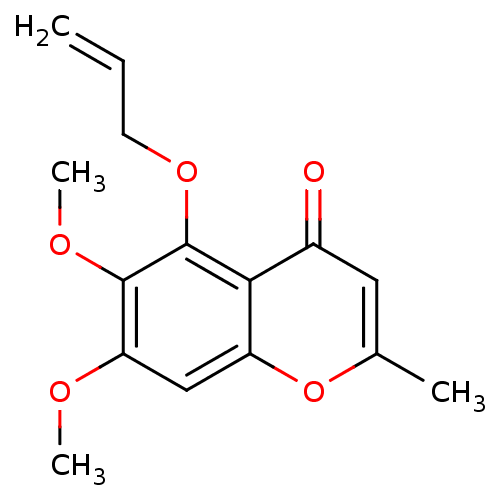
(6,7-dimethoxy-2-methyl-5-(prop-2-en-1-yloxy)-4H-ch...)Show InChI InChI=1S/C15H16O5/c1-5-6-19-15-13-10(16)7-9(2)20-11(13)8-12(17-3)14(15)18-4/h5,7-8H,1,6H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338657

(5-hydroxy-2-methyl-6,7-bis(prop-2-en-1-yloxy)-4H-c...)Show InChI InChI=1S/C16H16O5/c1-4-6-19-13-9-12-14(11(17)8-10(3)21-12)15(18)16(13)20-7-5-2/h4-5,8-9,18H,1-2,6-7H2,3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338661

(CHEMBL1684136 | Stellatin)Show InChI InChI=1S/C12H12O5/c1-6-4-7(13)10-8(17-6)5-9(15-2)12(16-3)11(10)14/h4-5,14H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338664

(6,7-dimethoxy-2-methyl-5-[(3-methylbut-2-en-1-yl)o...)Show SMILES [#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8]-[#6] Show InChI InChI=1S/C17H20O5/c1-10(2)6-7-21-17-15-12(18)8-11(3)22-13(15)9-14(19-4)16(17)20-5/h6,8-9H,7H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338660
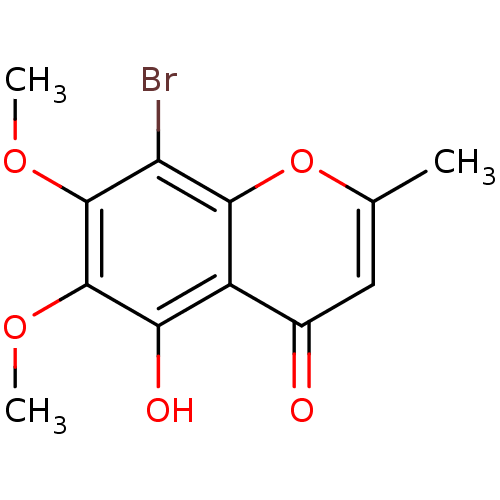
(8-bromo-5-hydroxy-6,7-dimethoxy-2-methyl-4H-chrome...)Show InChI InChI=1S/C12H11BrO5/c1-5-4-6(14)7-9(15)12(17-3)11(16-2)8(13)10(7)18-5/h4,15H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338667

(5-hydroxy-7-methoxy-2-methyl-6-(prop-2-en-1-yloxy)...)Show InChI InChI=1S/C14H14O5/c1-4-5-18-14-11(17-3)7-10-12(13(14)16)9(15)6-8(2)19-10/h4,6-7,16H,1,5H2,2-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338659
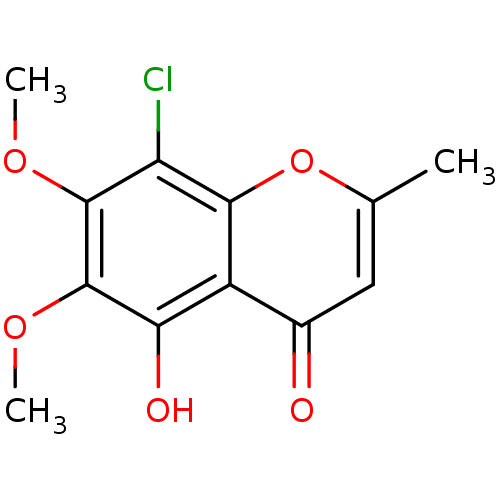
(8-chloro-5-hydroxy-6,7-dimethoxy-2-methyl-4H-chrom...)Show InChI InChI=1S/C12H11ClO5/c1-5-4-6(14)7-9(15)12(17-3)11(16-2)8(13)10(7)18-5/h4,15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338660

(8-bromo-5-hydroxy-6,7-dimethoxy-2-methyl-4H-chrome...)Show InChI InChI=1S/C12H11BrO5/c1-5-4-6(14)7-9(15)12(17-3)11(16-2)8(13)10(7)18-5/h4,15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50067040
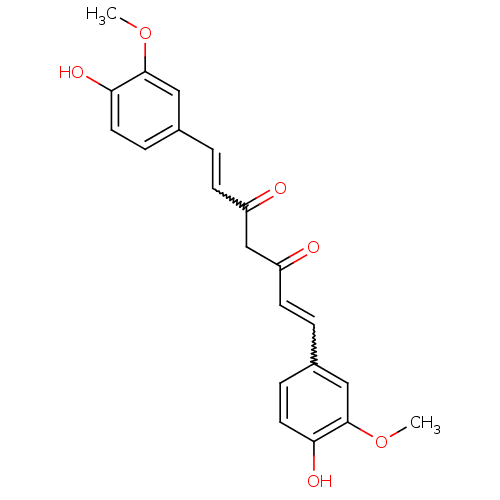
(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338663

(6,7-dimethoxy-2-methyl-5-(prop-2-en-1-yloxy)-4H-ch...)Show InChI InChI=1S/C15H16O5/c1-5-6-19-15-13-10(16)7-9(2)20-11(13)8-12(17-3)14(15)18-4/h5,7-8H,1,6H2,2-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338659

(8-chloro-5-hydroxy-6,7-dimethoxy-2-methyl-4H-chrom...)Show InChI InChI=1S/C12H11ClO5/c1-5-4-6(14)7-9(15)12(17-3)11(16-2)8(13)10(7)18-5/h4,15H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067040

(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data