

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339570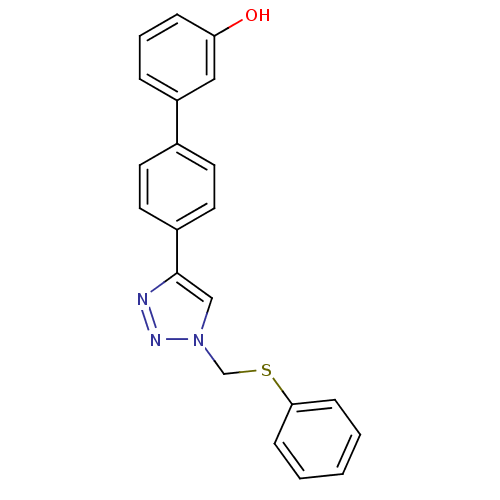 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339571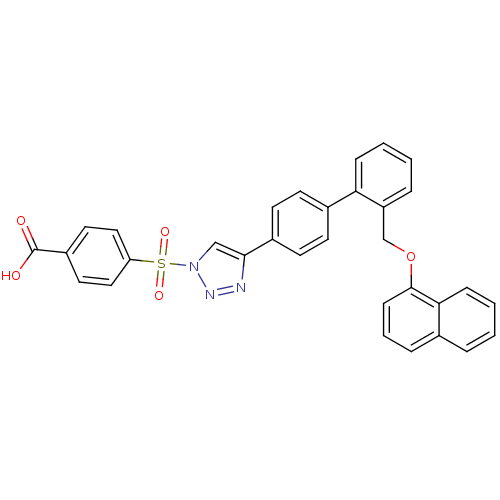 (4-{4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339571 (4-{4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339572 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339573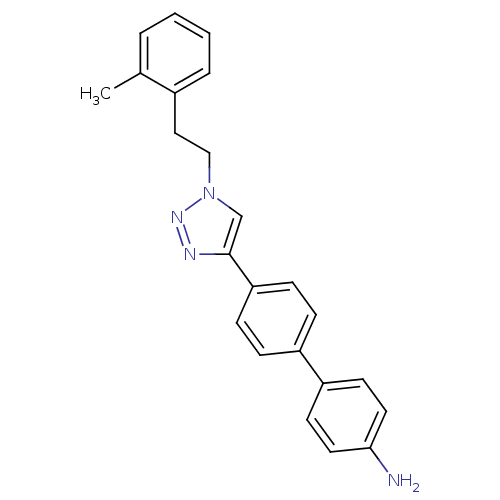 (4'-[1-(2-o-Tolyl-ethyl)-1H-[1,2,3]triazol-4-yl]-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339574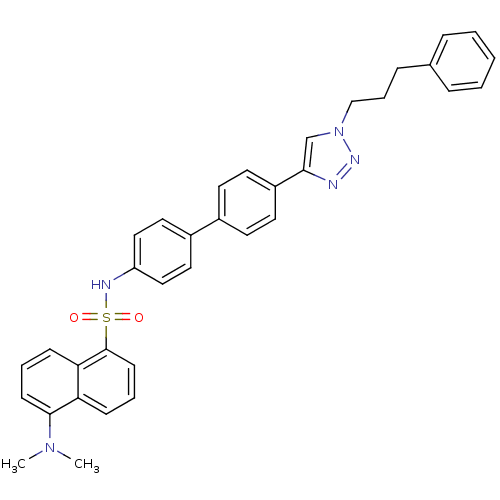 (5-Dimethylamino-naphthalene-1-sulfonic acid{4'-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339575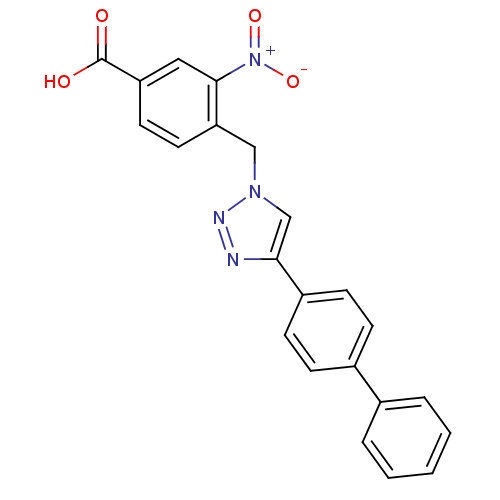 (4-(4-Biphenyl-4-yl-[1,2,3]triazol-1-ylmethyl)-3-ni...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339574 (5-Dimethylamino-naphthalene-1-sulfonic acid{4'-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339576 (4-(5-Benzyl-3-phenylsulfanylmethyl-3H-[1,2,3]triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339575 (4-(4-Biphenyl-4-yl-[1,2,3]triazol-1-ylmethyl)-3-ni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339577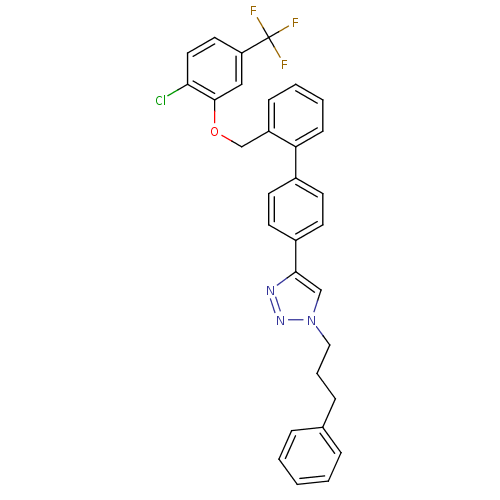 (4-[2'-(2-Chloro-5-trifluoromethyl-phenoxymethyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339575 (4-(4-Biphenyl-4-yl-[1,2,3]triazol-1-ylmethyl)-3-ni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339578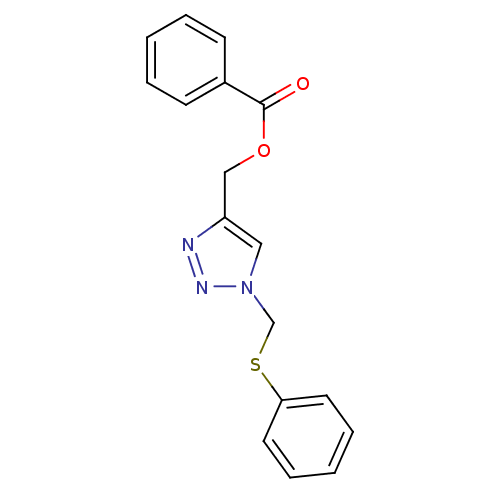 (Benzoic acid 1-Phenylsulfanylmethyl-1H-[1,2,3]Tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339570 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339572 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339579 (4-(4-Phenanthren-9-yl-phenyl)-1-(3-phenyl-propyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339578 (Benzoic acid 1-Phenylsulfanylmethyl-1H-[1,2,3]Tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339580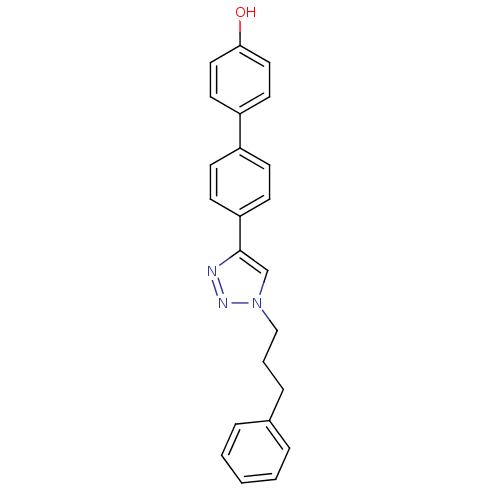 (4'-[1-(3-Phenyl-propyl)-1H-[1,2,3]triazol-4-yl]-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339581 (4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339582 (4-[2'-(2-Chloro-5-trifluoromethyl-phenoxymethyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339583 (4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339576 (4-(5-Benzyl-3-phenylsulfanylmethyl-3H-[1,2,3]triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339573 (4'-[1-(2-o-Tolyl-ethyl)-1H-[1,2,3]triazol-4-yl]-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339584 (4-(4'-Phenoxy-biphenyl-4-yl)-1-(3-phenyl-propyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipooxygenase expressed in Escherichia coli MV1190 by cell free assay | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339579 (4-(4-Phenanthren-9-yl-phenyl)-1-(3-phenyl-propyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339580 (4'-[1-(3-Phenyl-propyl)-1H-[1,2,3]triazol-4-yl]-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339581 (4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339577 (4-[2'-(2-Chloro-5-trifluoromethyl-phenoxymethyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339582 (4-[2'-(2-Chloro-5-trifluoromethyl-phenoxymethyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339583 (4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339584 (4-(4'-Phenoxy-biphenyl-4-yl)-1-(3-phenyl-propyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339573 (4'-[1-(2-o-Tolyl-ethyl)-1H-[1,2,3]triazol-4-yl]-bi...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339576 (4-(5-Benzyl-3-phenylsulfanylmethyl-3H-[1,2,3]triaz...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339583 (4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]-1-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339574 (5-Dimethylamino-naphthalene-1-sulfonic acid{4'-[1-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50339584 (4-(4'-Phenoxy-biphenyl-4-yl)-1-(3-phenyl-propyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339582 (4-[2'-(2-Chloro-5-trifluoromethyl-phenoxymethyl)-b...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339577 (4-[2'-(2-Chloro-5-trifluoromethyl-phenoxymethyl)-b...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339581 (4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]-1-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339580 (4'-[1-(3-Phenyl-propyl)-1H-[1,2,3]triazol-4-yl]-bi...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339578 (Benzoic acid 1-Phenylsulfanylmethyl-1H-[1,2,3]Tria...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339579 (4-(4-Phenanthren-9-yl-phenyl)-1-(3-phenyl-propyl)-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339570 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339572 (4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM50339571 (4-{4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Inhibition of Prostaglandin E2 synthase-1 in IL-1beta stimulated microsomal fraction of human A549 cell assessed as PGE2 level by RP-HPLC | J Med Chem 54: 1565-75 (2011) Article DOI: 10.1021/jm101238d BindingDB Entry DOI: 10.7270/Q2SN098M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||