

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79MZ cells using [1,2-3H]11-deoxycorticosterone/11-deoxycorticosterone | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in Chinese hamster V79MZ cells using [1,2-3H]11-deoxycorticosterone/11-deoxycorticosterone | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339655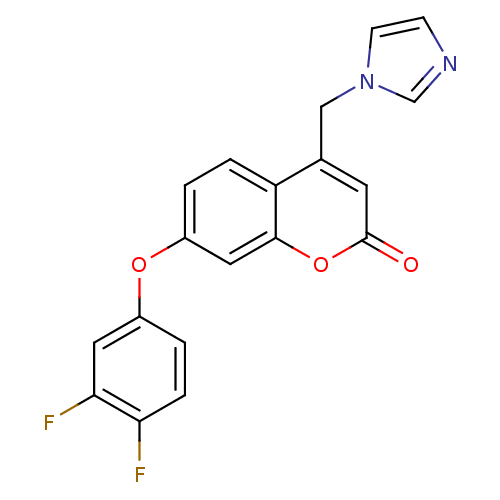 (7-(3,4-Difluorophenoxy)-4-(1H-imidazol-1-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9475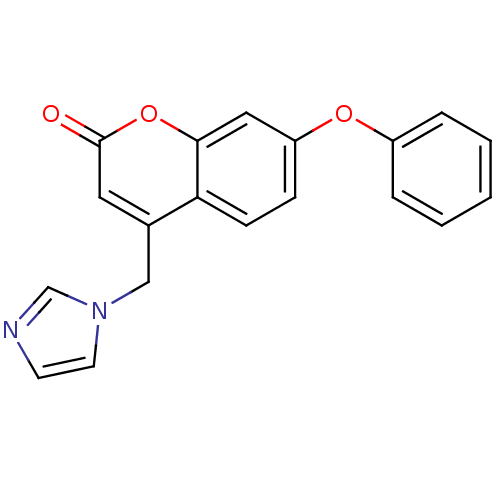 (4-(1H-Imidazol-1-ylmethyl)-7-phenoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339659 (CHEMBL1688915 | rac-4-[1H-Imidazol-1-yl(phenyl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339654 (7-(3,5-Difluorophenoxy)-4-(1H-imidazol-1-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339646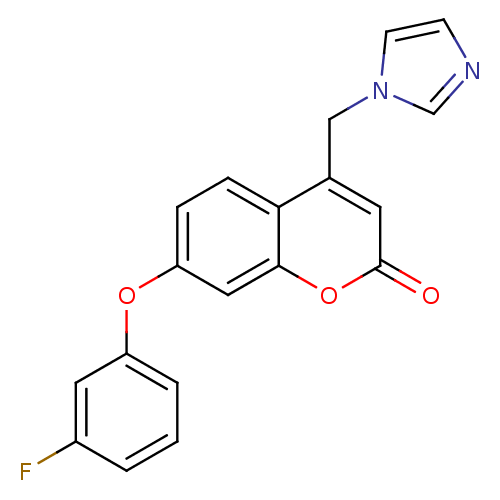 (7-(3-Fluorophenoxy)-4-(1H-imidazol-1-ylmethyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM9485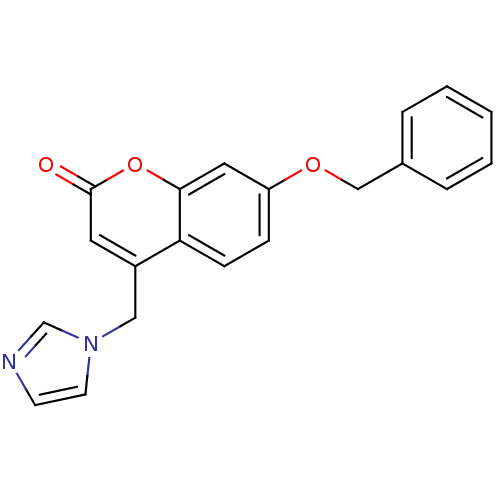 (7-(benzyloxy)-4-(1H-imidazol-1-ylmethyl)-2H-chrome...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in Chinese hamster V79MZ cells using [1,2-3H]11-deoxycorticosterone/11-deoxycorticosterone | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339647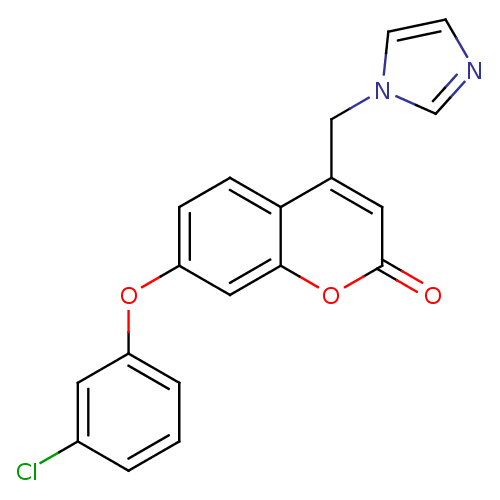 (7-(3-Chlorophenoxy)-4-(1H-imidazol-1-ylmethyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339653 (7-[4-(Dimethylamino)phenoxy]-4-(1H-imidazol-1-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339656 (7-Anilino-4-(1H-imidazol-1-ylmethyl)-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339650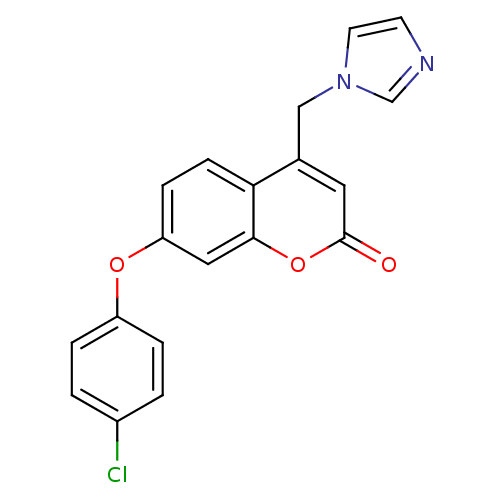 (7-(4-Chlorophenoxy)-4-(1H-imidazol-1-ylmethyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339665 (7-[(3-Fluorobenzyl)oxy]-4-(1H-imidazol-1-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339664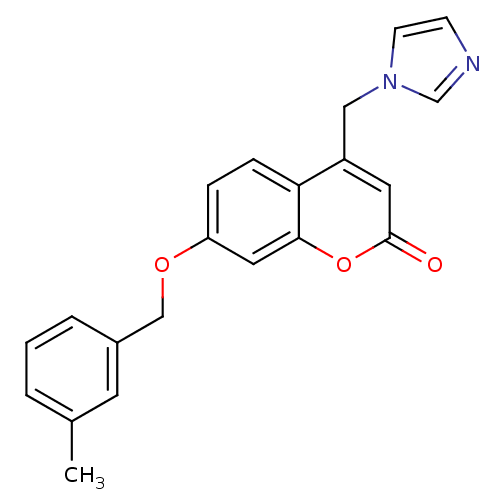 (4-(1H-Imidazol-1-ylmethyl)-7-[(3-methylbenzyl)oxy]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339642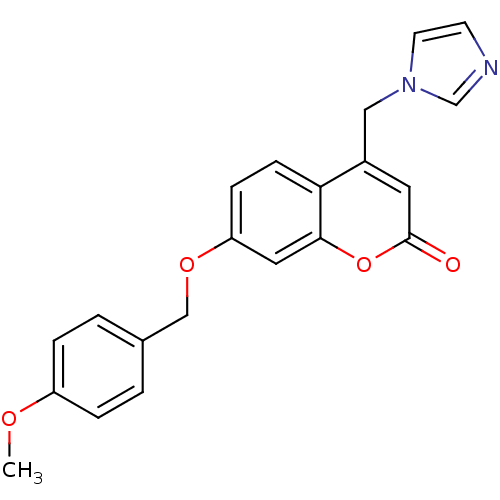 (4-(1H-Imidazol-1-ylmethyl)-7-[(4-methoxybenzyl)oxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339666 (7-[(3-Chlorobenzyl)oxy]-4-(1H-imidazol-1-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339669 (4-(1H-Imidazol-1-ylmethyl)-7-[(3-nitrobenzyl)oxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9485 (7-(benzyloxy)-4-(1H-imidazol-1-ylmethyl)-2H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339651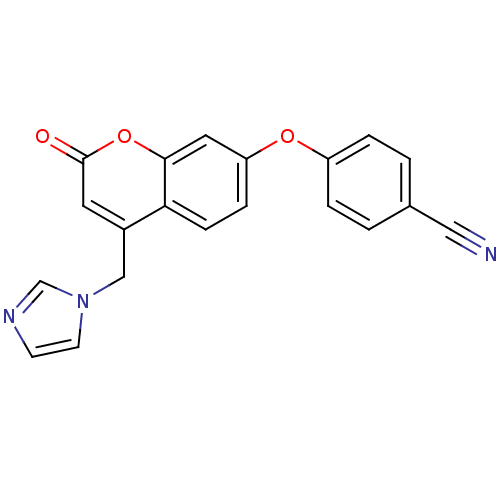 (4-{[4-(1H-Imidazol-1-ylmethyl)-2-oxo-2H-chromen-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339645 (7-[(3,4-Difluorobenzyl)oxy]-4-(1H-imidazol-1-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339644 (7-[(3,5-Difluorobenzyl)oxy]-4-(1H-imidazol-1-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339641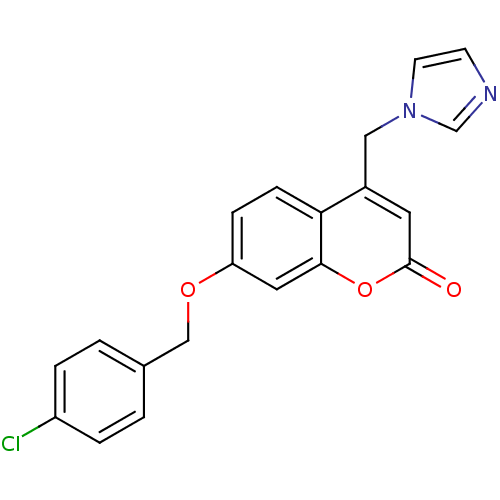 (7-[(4-Chlorobenzyl)oxy]-4-(1H-imidazol-1-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339668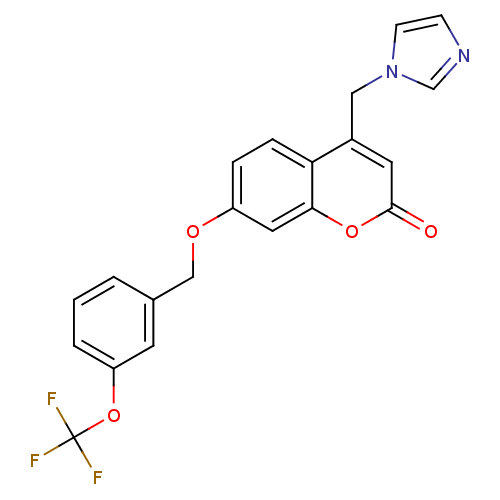 (4-(1H-Imidazol-1-ylmethyl)-7-{[3-(trifluoromethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339667 (4-(1H-Imidazol-1-ylmethyl)-7-{[3-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339640 (7-[(4-Fluorobenzyl)oxy]-4-(1H-imidazol-1-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9471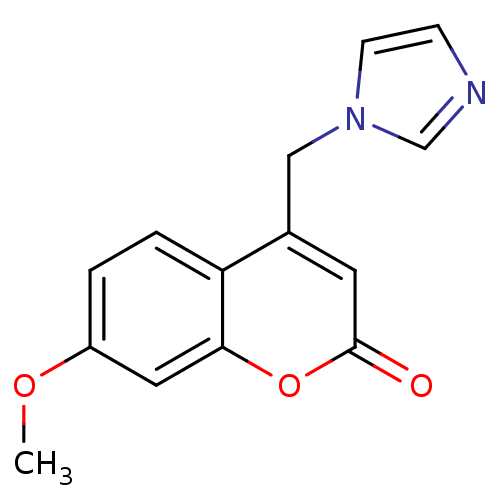 (4-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM9485 (7-(benzyloxy)-4-(1H-imidazol-1-ylmethyl)-2H-chrome...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 289 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79MZ cells using [1,2-3H]11-deoxycorticosterone/11-deoxycorticosterone | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339648 (4-(1H-Imidazol-1-ylmethyl)-7-(3-methoxyphenoxy)-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 292 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339652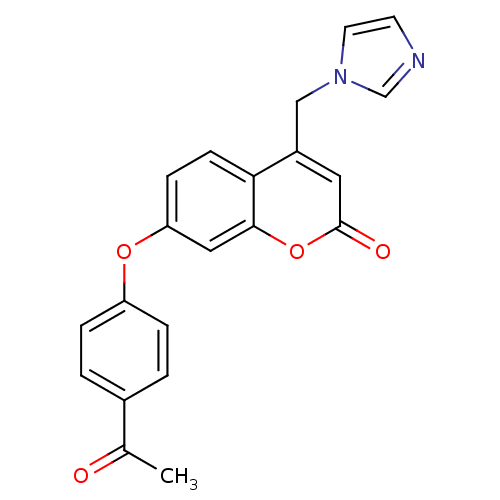 (7-(4-Acetylphenoxy)-4-(1H-imidazol-1-ylmethyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339663 (CHEMBL1688919 | rac-3-[1H-Imidazol-1-yl(phenyl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339660 (CHEMBL1688916 | rac-7-(3,4-Difluorophenoxy)-4-[1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 317 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339658 (CHEMBL1688914 | rac-4-[1H-Imidazol-1-yl(phenyl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339643 (4-(1H-Imidazol-1-ylmethyl)-7-[(4-trifluoromethoxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 481 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339661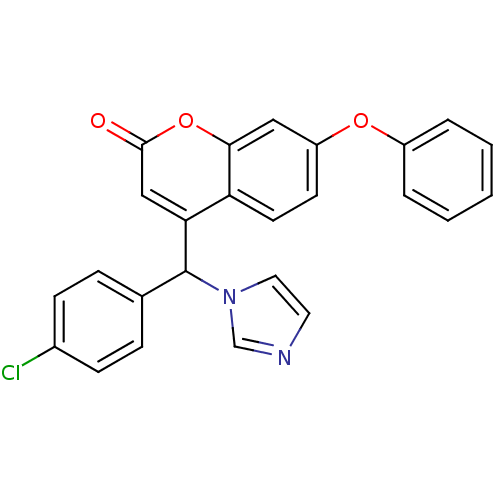 (CHEMBL1688917 | rac-4-[(4-Chlorophenyl)(1H-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 532 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339649 (4-(1H-Imidazol-1-ylmethyl)-7-(4-methylphenoxy)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50339655 (7-(3,4-Difluorophenoxy)-4-(1H-imidazol-1-ylmethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 933 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in Chinese hamster V79MZ cells using [1,2-3H]11-deoxycorticosterone/11-deoxycorticosterone | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50339655 (7-(3,4-Difluorophenoxy)-4-(1H-imidazol-1-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79MZ cells using [1,2-3H]11-deoxycorticosterone/11-deoxycorticosterone | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9469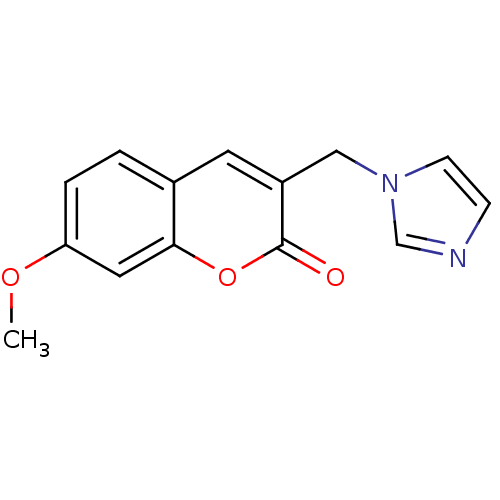 (3-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339657 (7-Hydroxy-4-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50339662 (CHEMBL1688918 | rac-4-[1H-Imidazol-1-yl(2-oxo-7-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human placental microsome CYP19 using [1beta-3H] androstenedione as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using progesterone as a substrate | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||