 Found 10 hits Enz. Inhib. hit(s) with all data for entry = 50034739
Found 10 hits Enz. Inhib. hit(s) with all data for entry = 50034739 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50403124

(CHEMBL2115571)Show SMILES C[S+](CCC(N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N6O5S/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/p+1/t7?,8-,10-,11-,14-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the bacterial AdoMet-DC; value ranges from 3.8 to 39.6 uM |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50281293
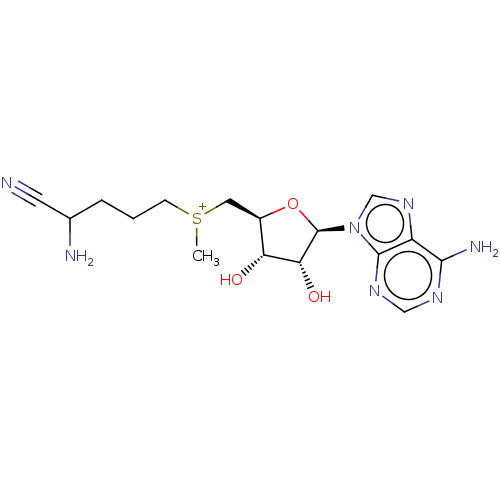
(2-amino-5-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...)Show SMILES Br.[Br-].C[S+](CCCC(N)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C16H24N7O3S/c1-27(4-2-3-9(18)5-17)6-10-12(24)13(25)16(26-10)23-8-22-11-14(19)20-7-21-15(11)23/h7-10,12-13,16,24-25H,2-4,6,18H2,1H3,(H2,19,20,21)/q+1/p+1/t9?,10-,12-,13-,16?,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the human AdoMet-DC |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50403124

(CHEMBL2115571)Show SMILES C[S+](CCC(N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N6O5S/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/p+1/t7?,8-,10-,11-,14-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the human AdoMet-DC; value ranges from 10.7 to 62.7 uM |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50403124

(CHEMBL2115571)Show SMILES C[S+](CCC(N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N6O5S/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/p+1/t7?,8-,10-,11-,14-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the bacterial AdoMet-DC; value ranges from 3.8 to 39.6 uM |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50281292
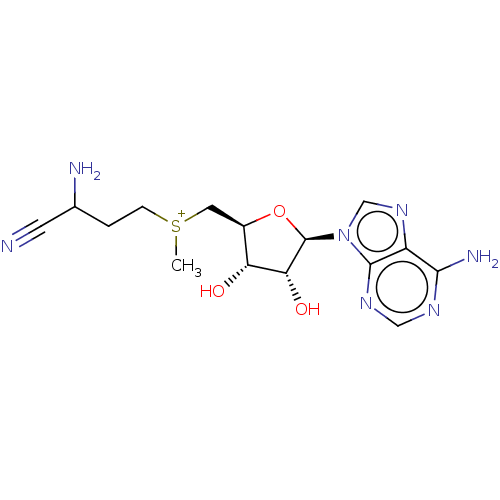
(2-amino-4-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...)Show SMILES Br.[Br-].C[S+](CCC(N)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N7O3S/c1-26(3-2-8(17)4-16)5-9-11(23)12(24)15(25-9)22-7-21-10-13(18)19-6-20-14(10)22/h6-9,11-12,15,23-24H,2-3,5,17H2,1H3,(H2,18,19,20)/q+1/p+1/t8?,9-,11-,12-,15?,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the bacterial AdoMet-DC |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50281292

(2-amino-4-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...)Show SMILES Br.[Br-].C[S+](CCC(N)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N7O3S/c1-26(3-2-8(17)4-16)5-9-11(23)12(24)15(25-9)22-7-21-10-13(18)19-6-20-14(10)22/h6-9,11-12,15,23-24H,2-3,5,17H2,1H3,(H2,18,19,20)/q+1/p+1/t8?,9-,11-,12-,15?,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the human AdoMet-DC |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50281292

(2-amino-4-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...)Show SMILES Br.[Br-].C[S+](CCC(N)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C15H22N7O3S/c1-26(3-2-8(17)4-16)5-9-11(23)12(24)15(25-9)22-7-21-10-13(18)19-6-20-14(10)22/h6-9,11-12,15,23-24H,2-3,5,17H2,1H3,(H2,18,19,20)/q+1/p+1/t8?,9-,11-,12-,15?,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against AdoMet-DC from Escherichia coli |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50281293

(2-amino-5-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...)Show SMILES Br.[Br-].C[S+](CCCC(N)C#N)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C16H24N7O3S/c1-27(4-2-3-9(18)5-17)6-10-12(24)13(25)16(26-10)23-8-22-11-14(19)20-7-21-15(11)23/h7-10,12-13,16,24-25H,2-4,6,18H2,1H3,(H2,19,20,21)/q+1/p+1/t9?,10-,12-,13-,16?,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated to inactivate the bacterial AdoMet-DC |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50366314
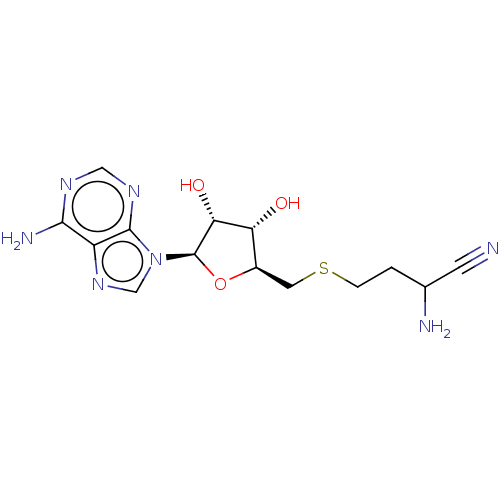
(CHEMBL3392211 | CHEMBL607699)Show SMILES NC(CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#N |r| Show InChI InChI=1S/C14H19N7O3S/c15-3-7(16)1-2-25-4-8-10(22)11(23)14(24-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,1-2,4,16H2,(H2,17,18,19)/t7?,8-,10-,11-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against AdoMet-DC from Escherichia coli |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine decarboxylase proenzyme
(Homo sapiens (Human)) | BDBM50366315
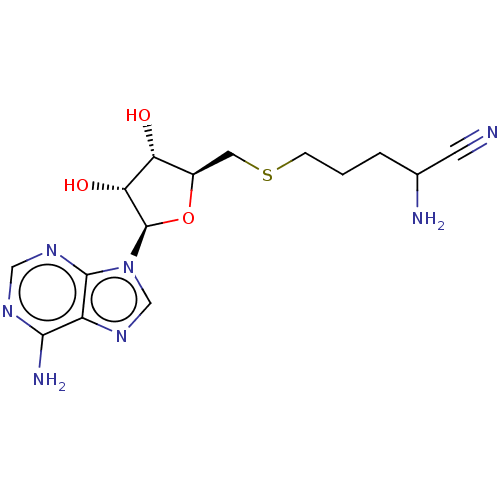
(CHEMBL1163096 | CHEMBL3392218)Show SMILES NC(CCCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#N |r| Show InChI InChI=1S/C15H21N7O3S/c16-4-8(17)2-1-3-26-5-9-11(23)12(24)15(25-9)22-7-21-10-13(18)19-6-20-14(10)22/h6-9,11-12,15,23-24H,1-3,5,17H2,(H2,18,19,20)/t8?,9-,11-,12-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against AdoMet-DC from Escherichia coli |
Bioorg Med Chem Lett 3: 2811-2816 (1993)
Article DOI: 10.1016/S0960-894X(01)80770-8
BindingDB Entry DOI: 10.7270/Q2TH8N6T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data