 Found 40 hits Enz. Inhib. hit(s) with all data for entry = 50036742
Found 40 hits Enz. Inhib. hit(s) with all data for entry = 50036742 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079861

((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H17FN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079861

((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H17FN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079861

((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H17FN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079866
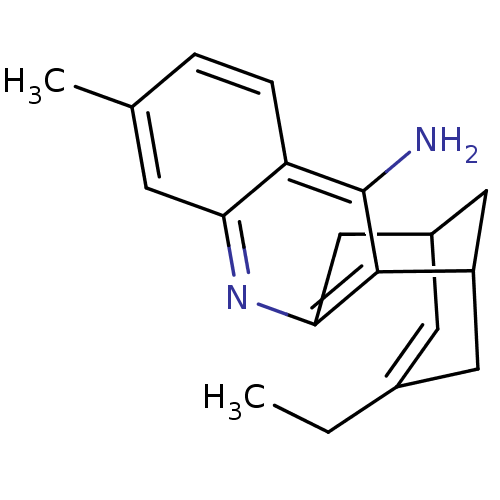
((+)-15-ethyl-7-methyl-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(C)ccc2c1N |t:2,TLB:19:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C19H22N2/c1-3-12-7-13-9-14(8-12)18-17(10-13)21-16-6-11(2)4-5-15(16)19(18)20/h4-7,13-14H,3,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079871
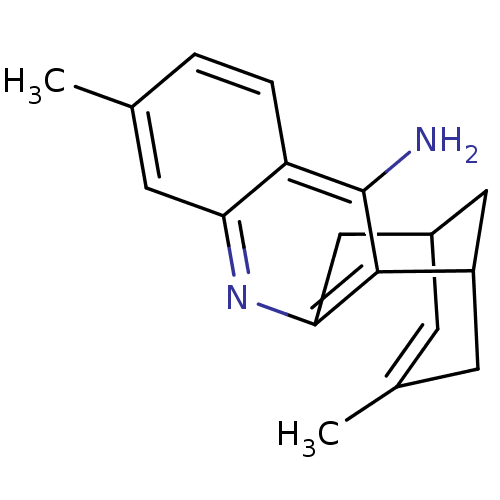
((rac)7,15-dimethyl-10-azatetracyclo[11.3.1.02,11.0...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(C)ccc2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C18H20N2/c1-10-3-4-14-15(7-10)20-16-9-12-5-11(2)6-13(8-12)17(16)18(14)19/h3-5,7,12-13H,6,8-9H2,1-2H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079866

((+)-15-ethyl-7-methyl-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(C)ccc2c1N |t:2,TLB:19:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C19H22N2/c1-3-12-7-13-9-14(8-12)18-17(10-13)21-16-6-11(2)4-5-15(16)19(18)20/h4-7,13-14H,3,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079866

((+)-15-ethyl-7-methyl-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(C)ccc2c1N |t:2,TLB:19:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C19H22N2/c1-3-12-7-13-9-14(8-12)18-17(10-13)21-16-6-11(2)4-5-15(16)19(18)20/h4-7,13-14H,3,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079869
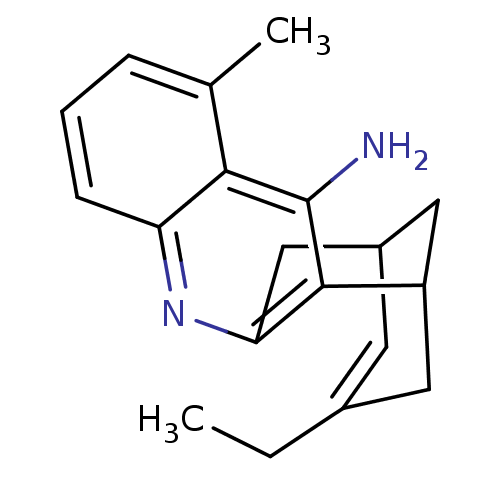
((rac)15-ethyl-5-methyl-10-azatetracyclo[11.3.1.02,...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cccc(C)c2c1N |t:2,TLB:19:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C19H22N2/c1-3-12-7-13-9-14(8-12)18-16(10-13)21-15-6-4-5-11(2)17(15)19(18)20/h4-7,13-14H,3,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079867
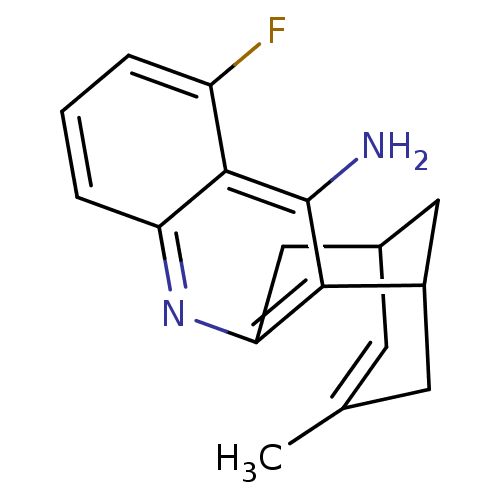
((rac)5-fluoro-15-methyl-10-azatetracyclo[11.3.1.02...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cccc(F)c2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H17FN2/c1-9-5-10-7-11(6-9)15-14(8-10)20-13-4-2-3-12(18)16(13)17(15)19/h2-5,10-11H,6-8H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079865
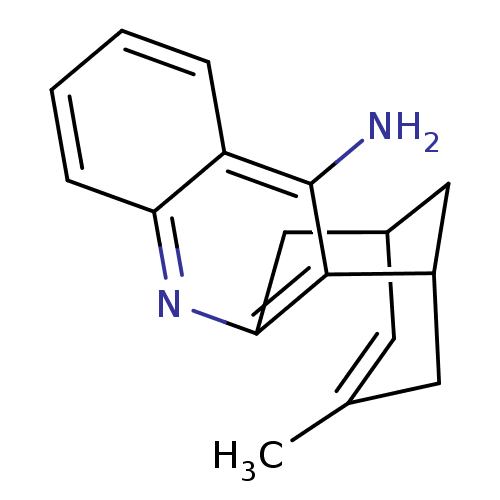
((+)-15-methyl-10-azatetracyclo[11.3.1.02,11.04,9]h...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:1,TLB:17:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H18N2/c1-10-6-11-8-12(7-10)16-15(9-11)19-14-5-3-2-4-13(14)17(16)18/h2-6,11-12H,7-9H2,1H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079865

((+)-15-methyl-10-azatetracyclo[11.3.1.02,11.04,9]h...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:1,TLB:17:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H18N2/c1-10-6-11-8-12(7-10)16-15(9-11)19-14-5-3-2-4-13(14)17(16)18/h2-6,11-12H,7-9H2,1H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079863
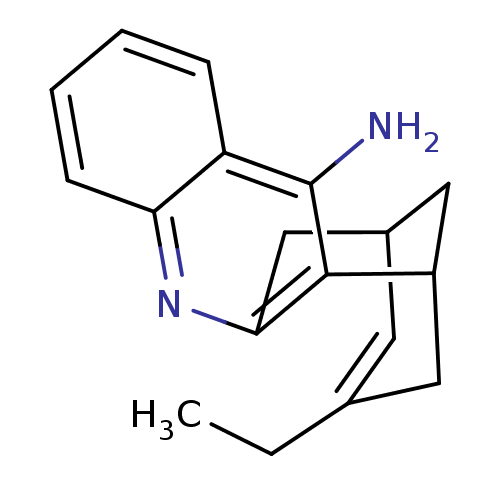
((+)-15-ethyl-10-azatetracyclo[11.3.1.02,11.04,9]he...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:2,TLB:18:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C18H20N2/c1-2-11-7-12-9-13(8-11)17-16(10-12)20-15-6-4-3-5-14(15)18(17)19/h3-7,12-13H,2,8-10H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079863

((+)-15-ethyl-10-azatetracyclo[11.3.1.02,11.04,9]he...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:2,TLB:18:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C18H20N2/c1-2-11-7-12-9-13(8-11)17-16(10-12)20-15-6-4-3-5-14(15)18(17)19/h3-7,12-13H,2,8-10H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079863

((+)-15-ethyl-10-azatetracyclo[11.3.1.02,11.04,9]he...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:2,TLB:18:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C18H20N2/c1-2-11-7-12-9-13(8-11)17-16(10-12)20-15-6-4-3-5-14(15)18(17)19/h3-7,12-13H,2,8-10H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079865

((+)-15-methyl-10-azatetracyclo[11.3.1.02,11.04,9]h...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:1,TLB:17:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H18N2/c1-10-6-11-8-12(7-10)16-15(9-11)19-14-5-3-2-4-13(14)17(16)18/h2-6,11-12H,7-9H2,1H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50199522

((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@@]1(N)CC(C)=C2 |r,c:18,THB:1:2:14.15.17:5.11.4| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079865

((+)-15-methyl-10-azatetracyclo[11.3.1.02,11.04,9]h...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:1,TLB:17:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H18N2/c1-10-6-11-8-12(7-10)16-15(9-11)19-14-5-3-2-4-13(14)17(16)18/h2-6,11-12H,7-9H2,1H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079865

((+)-15-methyl-10-azatetracyclo[11.3.1.02,11.04,9]h...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:1,TLB:17:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H18N2/c1-10-6-11-8-12(7-10)16-15(9-11)19-14-5-3-2-4-13(14)17(16)18/h2-6,11-12H,7-9H2,1H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079864
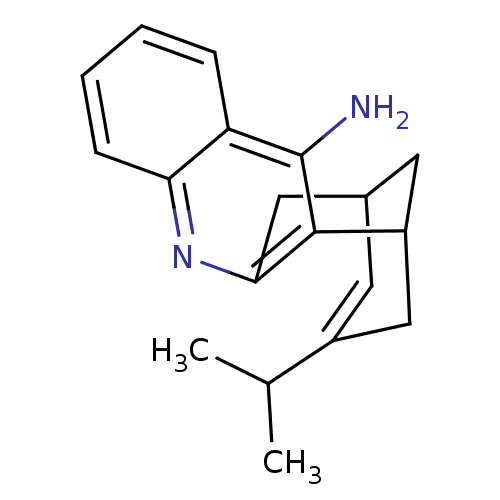
((rac)15-isopropyl-10-azatetracyclo[11.3.1.02,11.04...)Show SMILES CC(C)C1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:3,TLB:19:9:3.4.8:6,THB:1:3:9.10.11:6| Show InChI InChI=1S/C19H22N2/c1-11(2)13-7-12-8-14(10-13)18-17(9-12)21-16-6-4-3-5-15(16)19(18)20/h3-7,11-12,14H,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079866

((+)-15-ethyl-7-methyl-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(C)ccc2c1N |t:2,TLB:19:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C19H22N2/c1-3-12-7-13-9-14(8-12)18-17(10-13)21-16-6-11(2)4-5-15(16)19(18)20/h4-7,13-14H,3,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079868

((rac)15-phenyl-10-azatetracyclo[11.3.1.02,11.04,9]...)Show SMILES Nc1c2C3CC(Cc2nc2ccccc12)C=C(C3)c1ccccc1 |c:18,TLB:1:2:16.15.17:4,THB:18:16:2.7.6:4| Show InChI InChI=1S/C22H20N2/c23-22-18-8-4-5-9-19(18)24-20-12-14-10-16(13-17(11-14)21(20)22)15-6-2-1-3-7-15/h1-10,14,17H,11-13H2,(H2,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079865

((+)-15-methyl-10-azatetracyclo[11.3.1.02,11.04,9]h...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:1,TLB:17:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H18N2/c1-10-6-11-8-12(7-10)16-15(9-11)19-14-5-3-2-4-13(14)17(16)18/h2-6,11-12H,7-9H2,1H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079861

((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H17FN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079872

((rac)15-allyl-10-azatetracyclo[11.3.1.02,11.04,9]h...)Show SMILES Nc1c2C3CC(Cc2nc2ccccc12)C=C(CC=C)C3 |t:18,TLB:1:2:16.15.20:4,THB:17:16:2.7.6:4| Show InChI InChI=1S/C19H20N2/c1-2-5-12-8-13-10-14(9-12)18-17(11-13)21-16-7-4-3-6-15(16)19(18)20/h2-4,6-8,13-14H,1,5,9-11H2,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079861

((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H17FN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079866

((+)-15-ethyl-7-methyl-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(C)ccc2c1N |t:2,TLB:19:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C19H22N2/c1-3-12-7-13-9-14(8-12)18-17(10-13)21-16-6-11(2)4-5-15(16)19(18)20/h4-7,13-14H,3,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079866

((+)-15-ethyl-7-methyl-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(C)ccc2c1N |t:2,TLB:19:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C19H22N2/c1-3-12-7-13-9-14(8-12)18-17(10-13)21-16-6-11(2)4-5-15(16)19(18)20/h4-7,13-14H,3,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079870
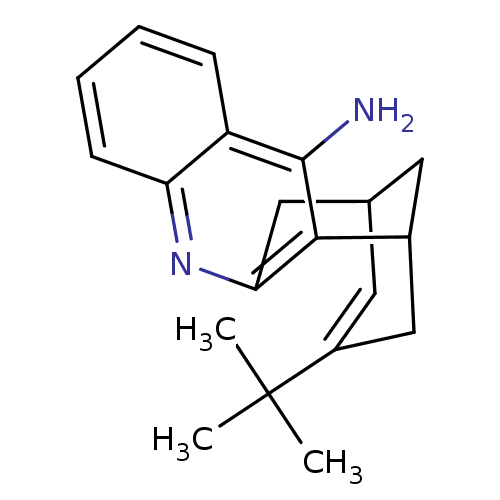
((rac)15-t-butyl-10-azatetracyclo[11.3.1.02,11.04,9...)Show SMILES CC(C)(C)C1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:4,TLB:20:10:4.5.9:7,THB:1:4:10.11.12:7| Show InChI InChI=1S/C20H24N2/c1-20(2,3)14-9-12-8-13(11-14)18-17(10-12)22-16-7-5-4-6-15(16)19(18)21/h4-7,9,12-13H,8,10-11H2,1-3H3,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079873
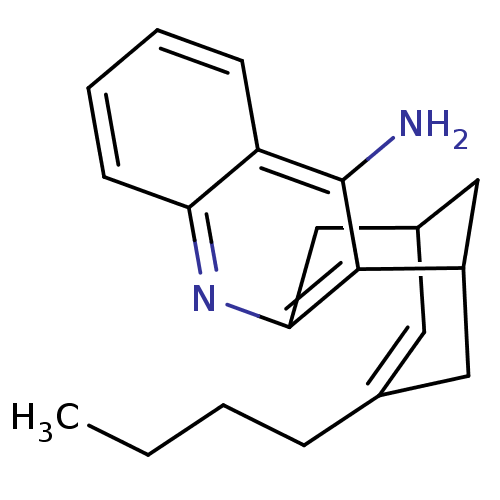
((rac)15-butyl-10-azatetracyclo[11.3.1.02,11.04,9]h...)Show SMILES CCCCC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:4,TLB:20:10:4.5.9:7,THB:3:4:10.11.12:7| Show InChI InChI=1S/C20H24N2/c1-2-3-6-13-9-14-11-15(10-13)19-18(12-14)22-17-8-5-4-7-16(17)20(19)21/h4-5,7-9,14-15H,2-3,6,10-12H2,1H3,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079862

((rac)15-propyl-10-azatetracyclo[11.3.1.02,11.04,9]...)Show SMILES CCCC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:3,TLB:19:9:3.4.8:6,THB:2:3:9.10.11:6| Show InChI InChI=1S/C19H22N2/c1-2-5-12-8-13-10-14(9-12)18-17(11-13)21-16-7-4-3-6-15(16)19(18)20/h3-4,6-8,13-14H,2,5,9-11H2,1H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079871

((rac)7,15-dimethyl-10-azatetracyclo[11.3.1.02,11.0...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(C)ccc2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C18H20N2/c1-10-3-4-14-15(7-10)20-16-9-12-5-11(2)6-13(8-12)17(16)18(14)19/h3-5,7,12-13H,6,8-9H2,1-2H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 449 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079869

((rac)15-ethyl-5-methyl-10-azatetracyclo[11.3.1.02,...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cccc(C)c2c1N |t:2,TLB:19:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C19H22N2/c1-3-12-7-13-9-14(8-12)18-16(10-13)21-15-6-4-5-11(2)17(15)19(18)20/h4-7,13-14H,3,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079867

((rac)5-fluoro-15-methyl-10-azatetracyclo[11.3.1.02...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cccc(F)c2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H17FN2/c1-9-5-10-7-11(6-9)15-14(8-10)20-13-4-2-3-12(18)16(13)17(15)19/h2-5,10-11H,6-8H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 543 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079863

((+)-15-ethyl-10-azatetracyclo[11.3.1.02,11.04,9]he...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:2,TLB:18:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C18H20N2/c1-2-11-7-12-9-13(8-11)17-16(10-12)20-15-6-4-3-5-14(15)18(17)19/h3-7,12-13H,2,8-10H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 888 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079863

((+)-15-ethyl-10-azatetracyclo[11.3.1.02,11.04,9]he...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:2,TLB:18:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C18H20N2/c1-2-11-7-12-9-13(8-11)17-16(10-12)20-15-6-4-3-5-14(15)18(17)19/h3-7,12-13H,2,8-10H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 888 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079863

((+)-15-ethyl-10-azatetracyclo[11.3.1.02,11.04,9]he...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:2,TLB:18:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C18H20N2/c1-2-11-7-12-9-13(8-11)17-16(10-12)20-15-6-4-3-5-14(15)18(17)19/h3-7,12-13H,2,8-10H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 888 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (AChE)activity in bovine erythrocytes |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50079861

((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H17FN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50199522

((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@@]1(N)CC(C)=C2 |r,c:18,THB:1:2:14.15.17:5.11.4| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of butyrylcholinesterase (BChE) activity in human serum |
J Med Chem 42: 3227-42 (1999)
Article DOI: 10.1021/jm980620z
BindingDB Entry DOI: 10.7270/Q2H70GH6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data