 Found 108 hits Enz. Inhib. hit(s) with all data for entry = 50038141
Found 108 hits Enz. Inhib. hit(s) with all data for entry = 50038141 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372118

(CHEMBL255389)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-10-6-5-7-11-23)17-18-39-26-12-8-9-22(19-26)20-27-29(32(37)38)35(31(27)36)25-15-13-24(14-16-25)33(2,3)4/h5-16,19,27,29H,17-18,20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372105

(CHEMBL411118)Show SMILES COc1ccc(cc1)N1[C@@H]([C@H](Cc2ccc(OCCc3nc(oc3C)-c3ccccc3)cc2)C1=O)C(O)=O Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-6-4-3-5-7-21)16-17-37-24-12-8-20(9-13-24)18-25-27(30(34)35)32(29(25)33)22-10-14-23(36-2)15-11-22/h3-15,25,27H,16-18H2,1-2H3,(H,34,35)/t25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372118

(CHEMBL255389)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-10-6-5-7-11-23)17-18-39-26-12-8-9-22(19-26)20-27-29(32(37)38)35(31(27)36)25-15-13-24(14-16-25)33(2,3)4/h5-16,19,27,29H,17-18,20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372107

(CHEMBL271240)Show SMILES COc1ccc(cc1)N1[C@@H]([C@H](Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O |r| Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-8-4-3-5-9-21)15-16-37-24-10-6-7-20(17-24)18-25-27(30(34)35)32(29(25)33)22-11-13-23(36-2)14-12-22/h3-14,17,25,27H,15-16,18H2,1-2H3,(H,34,35)/t25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372116
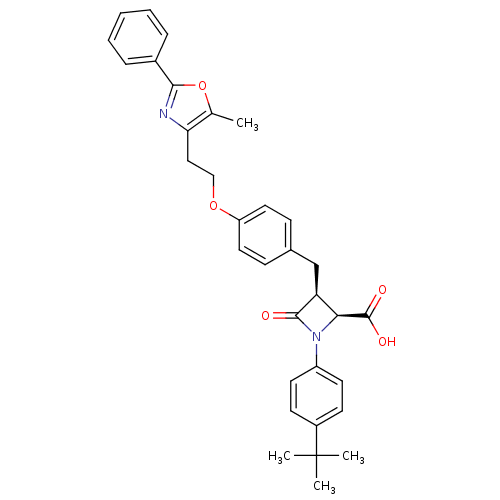
(CHEMBL256468)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-8-6-5-7-9-23)18-19-39-26-16-10-22(11-17-26)20-27-29(32(37)38)35(31(27)36)25-14-12-24(13-15-25)33(2,3)4/h5-17,27,29H,18-20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372114
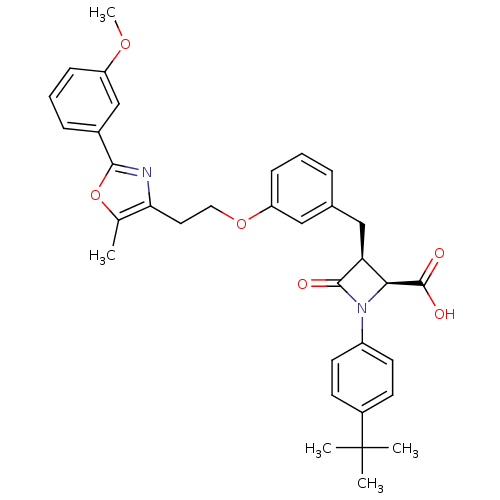
(CHEMBL404646)Show SMILES COc1cccc(c1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-7-10-26(20-23)40-5)16-17-41-27-11-6-8-22(18-27)19-28-30(33(38)39)36(32(28)37)25-14-12-24(13-15-25)34(2,3)4/h6-15,18,20,28,30H,16-17,19H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372106

(CHEMBL257891)Show SMILES COc1ccc(cc1)N1[C@H]([C@@H](Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-8-4-3-5-9-21)15-16-37-24-10-6-7-20(17-24)18-25-27(30(34)35)32(29(25)33)22-11-13-23(36-2)14-12-22/h3-14,17,25,27H,15-16,18H2,1-2H3,(H,34,35)/t25-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372110

(CHEMBL271739)Show SMILES Cc1oc(nc1CCOc1cccc(CC2C(N(C2=O)c2ccc(F)cc2)C(O)=O)c1)-c1ccccc1 |w:16.29,15.15| Show InChI InChI=1S/C29H25FN2O5/c1-18-25(31-27(37-18)20-7-3-2-4-8-20)14-15-36-23-9-5-6-19(16-23)17-24-26(29(34)35)32(28(24)33)22-12-10-21(30)11-13-22/h2-13,16,24,26H,14-15,17H2,1H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372112
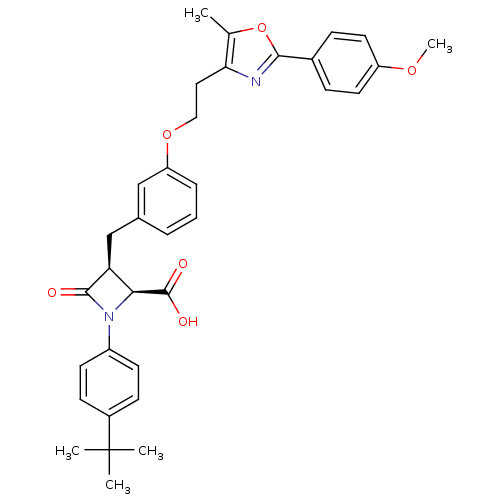
(CHEMBL257517)Show SMILES COc1ccc(cc1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-15-26(40-5)16-10-23)17-18-41-27-8-6-7-22(19-27)20-28-30(33(38)39)36(32(28)37)25-13-11-24(12-14-25)34(2,3)4/h6-16,19,28,30H,17-18,20H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50372118

(CHEMBL255389)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-10-6-5-7-11-23)17-18-39-26-12-8-9-22(19-26)20-27-29(32(37)38)35(31(27)36)25-15-13-24(14-16-25)33(2,3)4/h5-16,19,27,29H,17-18,20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372108

(CHEMBL272962)Show SMILES COc1ccc(cn1)N1C(C(Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O |w:9.39,10.11| Show InChI InChI=1S/C29H27N3O6/c1-18-24(31-27(38-18)20-8-4-3-5-9-20)13-14-37-22-10-6-7-19(15-22)16-23-26(29(34)35)32(28(23)33)21-11-12-25(36-2)30-17-21/h3-12,15,17,23,26H,13-14,16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372113

(CHEMBL429734)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1cccc(Cl)c1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-6-9-24(34)19-22)15-16-40-26-10-5-7-21(17-26)18-27-29(32(38)39)36(31(27)37)25-13-11-23(12-14-25)33(2,3)4/h5-14,17,19,27,29H,15-16,18H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372107

(CHEMBL271240)Show SMILES COc1ccc(cc1)N1[C@@H]([C@H](Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O |r| Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-8-4-3-5-9-21)15-16-37-24-10-6-7-20(17-24)18-25-27(30(34)35)32(29(25)33)22-11-13-23(36-2)14-12-22/h3-14,17,25,27H,15-16,18H2,1-2H3,(H,34,35)/t25-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372109

(CHEMBL255388)Show SMILES Cc1oc(nc1CCOc1cccc(CC2C(N(C2=O)c2ccc(Cl)cc2)C(O)=O)c1)-c1ccccc1 |w:16.29,15.15| Show InChI InChI=1S/C29H25ClN2O5/c1-18-25(31-27(37-18)20-7-3-2-4-8-20)14-15-36-23-9-5-6-19(16-23)17-24-26(29(34)35)32(28(24)33)22-12-10-21(30)11-13-22/h2-13,16,24,26H,14-15,17H2,1H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50372111
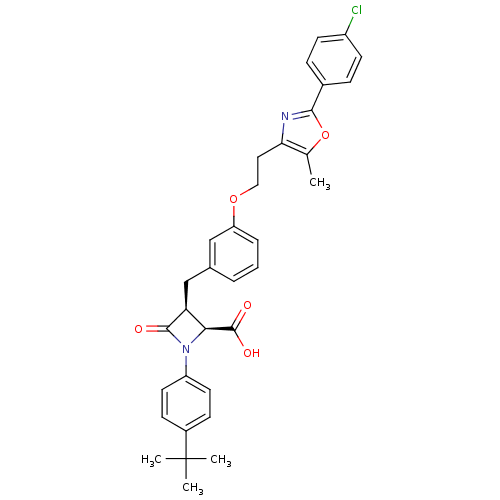
(CHEMBL272336)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-12-24(34)13-9-22)16-17-40-26-7-5-6-21(18-26)19-27-29(32(38)39)36(31(27)37)25-14-10-23(11-15-25)33(2,3)4/h5-15,18,27,29H,16-17,19H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372112

(CHEMBL257517)Show SMILES COc1ccc(cc1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-15-26(40-5)16-10-23)17-18-41-27-8-6-7-22(19-27)20-28-30(33(38)39)36(32(28)37)25-13-11-24(12-14-25)34(2,3)4/h6-16,19,28,30H,17-18,20H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372111

(CHEMBL272336)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-12-24(34)13-9-22)16-17-40-26-7-5-6-21(18-26)19-27-29(32(38)39)36(31(27)37)25-14-10-23(11-15-25)33(2,3)4/h5-15,18,27,29H,16-17,19H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372114

(CHEMBL404646)Show SMILES COc1cccc(c1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-7-10-26(20-23)40-5)16-17-41-27-11-6-8-22(18-27)19-28-30(33(38)39)36(32(28)37)25-14-12-24(13-15-25)34(2,3)4/h6-15,18,20,28,30H,16-17,19H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50372112

(CHEMBL257517)Show SMILES COc1ccc(cc1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-15-26(40-5)16-10-23)17-18-41-27-8-6-7-22(19-27)20-28-30(33(38)39)36(32(28)37)25-13-11-24(12-14-25)34(2,3)4/h6-16,19,28,30H,17-18,20H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372119

(CHEMBL271241)Show SMILES CCCCCCCN(CCc1ccc(O[C@](C)(CC)C(O)=O)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C27H36F2N2O4/c1-4-6-7-8-9-17-31(26(34)30-24-15-12-21(28)19-23(24)29)18-16-20-10-13-22(14-11-20)35-27(3,5-2)25(32)33/h10-15,19H,4-9,16-18H2,1-3H3,(H,30,34)(H,32,33)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50372114

(CHEMBL404646)Show SMILES COc1cccc(c1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-7-10-26(20-23)40-5)16-17-41-27-11-6-8-22(18-27)19-28-30(33(38)39)36(32(28)37)25-14-12-24(13-15-25)34(2,3)4/h6-15,18,20,28,30H,16-17,19H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50372117
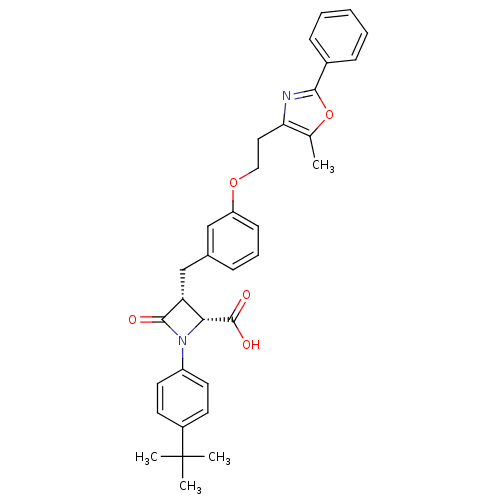
(CHEMBL272963)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2[C@@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-10-6-5-7-11-23)17-18-39-26-12-8-9-22(19-26)20-27-29(32(37)38)35(31(27)36)25-15-13-24(14-16-25)33(2,3)4/h5-16,19,27,29H,17-18,20H2,1-4H3,(H,37,38)/t27-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372111

(CHEMBL272336)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-12-24(34)13-9-22)16-17-40-26-7-5-6-21(18-26)19-27-29(32(38)39)36(31(27)37)25-14-10-23(11-15-25)33(2,3)4/h5-15,18,27,29H,16-17,19H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50372113

(CHEMBL429734)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1cccc(Cl)c1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-6-9-24(34)19-22)15-16-40-26-10-5-7-21(17-26)18-27-29(32(38)39)36(31(27)37)25-13-11-23(12-14-25)33(2,3)4/h5-14,17,19,27,29H,15-16,18H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372106

(CHEMBL257891)Show SMILES COc1ccc(cc1)N1[C@H]([C@@H](Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-8-4-3-5-9-21)15-16-37-24-10-6-7-20(17-24)18-25-27(30(34)35)32(29(25)33)22-11-13-23(36-2)14-12-22/h3-14,17,25,27H,15-16,18H2,1-2H3,(H,34,35)/t25-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372105

(CHEMBL411118)Show SMILES COc1ccc(cc1)N1[C@@H]([C@H](Cc2ccc(OCCc3nc(oc3C)-c3ccccc3)cc2)C1=O)C(O)=O Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-6-4-3-5-7-21)16-17-37-24-12-8-20(9-13-24)18-25-27(30(34)35)32(29(25)33)22-10-14-23(36-2)15-11-22/h3-15,25,27H,16-18H2,1-2H3,(H,34,35)/t25-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372119

(CHEMBL271241)Show SMILES CCCCCCCN(CCc1ccc(O[C@](C)(CC)C(O)=O)cc1)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C27H36F2N2O4/c1-4-6-7-8-9-17-31(26(34)30-24-15-12-21(28)19-23(24)29)18-16-20-10-13-22(14-11-20)35-27(3,5-2)25(32)33/h10-15,19H,4-9,16-18H2,1-3H3,(H,30,34)(H,32,33)/t27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372116

(CHEMBL256468)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-8-6-5-7-9-23)18-19-39-26-16-10-22(11-17-26)20-27-29(32(37)38)35(31(27)36)25-14-12-24(13-15-25)33(2,3)4/h5-17,27,29H,18-20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372117

(CHEMBL272963)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2[C@@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-10-6-5-7-11-23)17-18-39-26-12-8-9-22(19-26)20-27-29(32(37)38)35(31(27)36)25-15-13-24(14-16-25)33(2,3)4/h5-16,19,27,29H,17-18,20H2,1-4H3,(H,37,38)/t27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50372107

(CHEMBL271240)Show SMILES COc1ccc(cc1)N1[C@@H]([C@H](Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O |r| Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-8-4-3-5-9-21)15-16-37-24-10-6-7-20(17-24)18-25-27(30(34)35)32(29(25)33)22-11-13-23(36-2)14-12-22/h3-14,17,25,27H,15-16,18H2,1-2H3,(H,34,35)/t25-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in presence of 7-benzyloxyresorufin substrate |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372113

(CHEMBL429734)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1cccc(Cl)c1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-6-9-24(34)19-22)15-16-40-26-10-5-7-21(17-26)18-27-29(32(38)39)36(31(27)37)25-13-11-23(12-14-25)33(2,3)4/h5-14,17,19,27,29H,15-16,18H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372106

(CHEMBL257891)Show SMILES COc1ccc(cc1)N1[C@H]([C@@H](Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-8-4-3-5-9-21)15-16-37-24-10-6-7-20(17-24)18-25-27(30(34)35)32(29(25)33)22-11-13-23(36-2)14-12-22/h3-14,17,25,27H,15-16,18H2,1-2H3,(H,34,35)/t25-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372108

(CHEMBL272962)Show SMILES COc1ccc(cn1)N1C(C(Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O |w:9.39,10.11| Show InChI InChI=1S/C29H27N3O6/c1-18-24(31-27(38-18)20-8-4-3-5-9-20)13-14-37-22-10-6-7-19(15-22)16-23-26(29(34)35)32(28(23)33)21-11-12-25(36-2)30-17-21/h3-12,15,17,23,26H,13-14,16H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50372111

(CHEMBL272336)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-12-24(34)13-9-22)16-17-40-26-7-5-6-21(18-26)19-27-29(32(38)39)36(31(27)37)25-14-10-23(11-15-25)33(2,3)4/h5-15,18,27,29H,16-17,19H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by FLIPR assay |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372109

(CHEMBL255388)Show SMILES Cc1oc(nc1CCOc1cccc(CC2C(N(C2=O)c2ccc(Cl)cc2)C(O)=O)c1)-c1ccccc1 |w:16.29,15.15| Show InChI InChI=1S/C29H25ClN2O5/c1-18-25(31-27(37-18)20-7-3-2-4-8-20)14-15-36-23-9-5-6-19(16-23)17-24-26(29(34)35)32(28(24)33)22-12-10-21(30)11-13-22/h2-13,16,24,26H,14-15,17H2,1H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50372113

(CHEMBL429734)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1cccc(Cl)c1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-6-9-24(34)19-22)15-16-40-26-10-5-7-21(17-26)18-27-29(32(38)39)36(31(27)37)25-13-11-23(12-14-25)33(2,3)4/h5-14,17,19,27,29H,15-16,18H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by FLIPR assay |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50372114

(CHEMBL404646)Show SMILES COc1cccc(c1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-7-10-26(20-23)40-5)16-17-41-27-11-6-8-22(18-27)19-28-30(33(38)39)36(32(28)37)25-14-12-24(13-15-25)34(2,3)4/h6-15,18,20,28,30H,16-17,19H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by FLIPR assay |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50372107

(CHEMBL271240)Show SMILES COc1ccc(cc1)N1[C@@H]([C@H](Cc2cccc(OCCc3nc(oc3C)-c3ccccc3)c2)C1=O)C(O)=O |r| Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-8-4-3-5-9-21)15-16-37-24-10-6-7-20(17-24)18-25-27(30(34)35)32(29(25)33)22-11-13-23(36-2)14-12-22/h3-14,17,25,27H,15-16,18H2,1-2H3,(H,34,35)/t25-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50372116

(CHEMBL256468)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-8-6-5-7-9-23)18-19-39-26-16-10-22(11-17-26)20-27-29(32(37)38)35(31(27)36)25-14-12-24(13-15-25)33(2,3)4/h5-17,27,29H,18-20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50372110

(CHEMBL271739)Show SMILES Cc1oc(nc1CCOc1cccc(CC2C(N(C2=O)c2ccc(F)cc2)C(O)=O)c1)-c1ccccc1 |w:16.29,15.15| Show InChI InChI=1S/C29H25FN2O5/c1-18-25(31-27(37-18)20-7-3-2-4-8-20)14-15-36-23-9-5-6-19(16-23)17-24-26(29(34)35)32(28(24)33)22-12-10-21(30)11-13-22/h2-13,16,24,26H,14-15,17H2,1H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARalpha |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50372111

(CHEMBL272336)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-12-24(34)13-9-22)16-17-40-26-7-5-6-21(18-26)19-27-29(32(38)39)36(31(27)37)25-14-10-23(11-15-25)33(2,3)4/h5-15,18,27,29H,16-17,19H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50372115
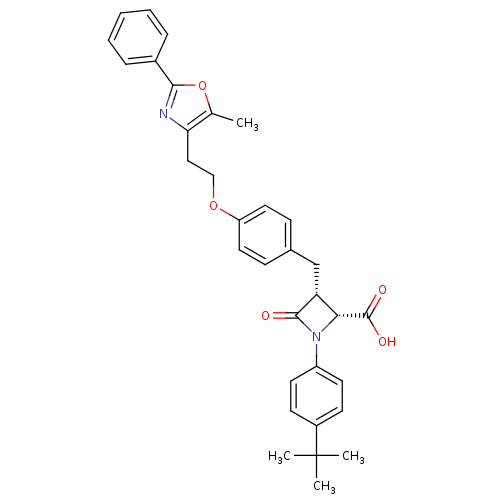
(CHEMBL404451)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2[C@@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-8-6-5-7-9-23)18-19-39-26-16-10-22(11-17-26)20-27-29(32(37)38)35(31(27)36)25-14-12-24(13-15-25)33(2,3)4/h5-17,27,29H,18-20H2,1-4H3,(H,37,38)/t27-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50372112

(CHEMBL257517)Show SMILES COc1ccc(cc1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-15-26(40-5)16-10-23)17-18-41-27-8-6-7-22(19-27)20-28-30(33(38)39)36(32(28)37)25-13-11-24(12-14-25)34(2,3)4/h6-16,19,28,30H,17-18,20H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by FLIPR assay |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372120

(CHEMBL272774)Show SMILES COc1ccc(cc1)N1[C@H]([C@@H](Cc2ccc(OCCc3nc(oc3C)-c3ccccc3)cc2)C1=O)C(O)=O Show InChI InChI=1S/C30H28N2O6/c1-19-26(31-28(38-19)21-6-4-3-5-7-21)16-17-37-24-12-8-20(9-13-24)18-25-27(30(34)35)32(29(25)33)22-10-14-23(36-2)15-11-22/h3-15,25,27H,16-18H2,1-2H3,(H,34,35)/t25-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50372115

(CHEMBL404451)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2[C@@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-8-6-5-7-9-23)18-19-39-26-16-10-22(11-17-26)20-27-29(32(37)38)35(31(27)36)25-14-12-24(13-15-25)33(2,3)4/h5-17,27,29H,18-20H2,1-4H3,(H,37,38)/t27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50372111

(CHEMBL272336)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-12-24(34)13-9-22)16-17-40-26-7-5-6-21(18-26)19-27-29(32(38)39)36(31(27)37)25-14-10-23(11-15-25)33(2,3)4/h5-15,18,27,29H,16-17,19H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in presence of 7-benzyloxy-4-trifluoromethyl coumarin substrate |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50372113

(CHEMBL429734)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1cccc(Cl)c1 Show InChI InChI=1S/C33H33ClN2O5/c1-20-28(35-30(41-20)22-8-6-9-24(34)19-22)15-16-40-26-10-5-7-21(17-26)18-27-29(32(38)39)36(31(27)37)25-13-11-23(12-14-25)33(2,3)4/h5-14,17,19,27,29H,15-16,18H2,1-4H3,(H,38,39)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50372118

(CHEMBL255389)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2[C@H](N(C2=O)c2ccc(cc2)C(C)(C)C)C(O)=O)c1)-c1ccccc1 Show InChI InChI=1S/C33H34N2O5/c1-21-28(34-30(40-21)23-10-6-5-7-11-23)17-18-39-26-12-8-9-22(19-26)20-27-29(32(37)38)35(31(27)36)25-15-13-24(14-16-25)33(2,3)4/h5-16,19,27,29H,17-18,20H2,1-4H3,(H,37,38)/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by FLIPR assay |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50372114

(CHEMBL404646)Show SMILES COc1cccc(c1)-c1nc(CCOc2cccc(C[C@H]3[C@H](N(C3=O)c3ccc(cc3)C(C)(C)C)C(O)=O)c2)c(C)o1 Show InChI InChI=1S/C34H36N2O6/c1-21-29(35-31(42-21)23-9-7-10-26(20-23)40-5)16-17-41-27-11-6-8-22(18-27)19-28-30(33(38)39)36(32(28)37)25-14-12-24(13-15-25)34(2,3)4/h6-15,18,20,28,30H,16-17,19H2,1-5H3,(H,38,39)/t28-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 18: 1939-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.126
BindingDB Entry DOI: 10.7270/Q28S4QR3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data