

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50013804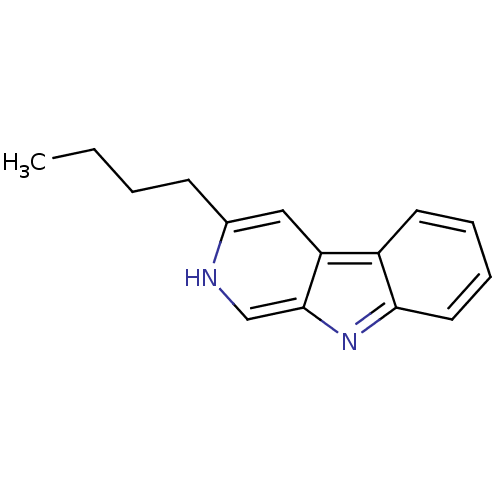 (10,13-Dimethyl-hexadecahydro-cyclopenta[a]phenanth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM36371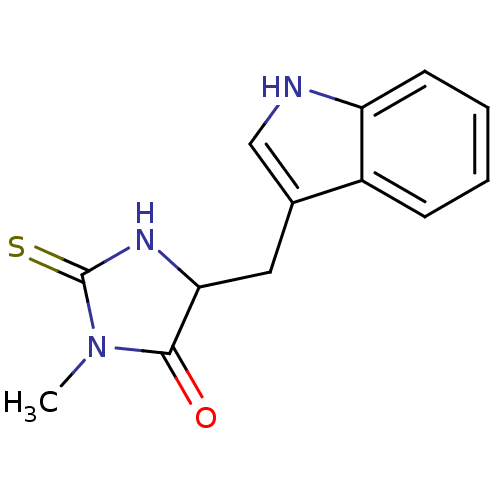 (5-(1H-indol-3-ylmethyl)-3-methyl-2-thioxo-4-Imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21973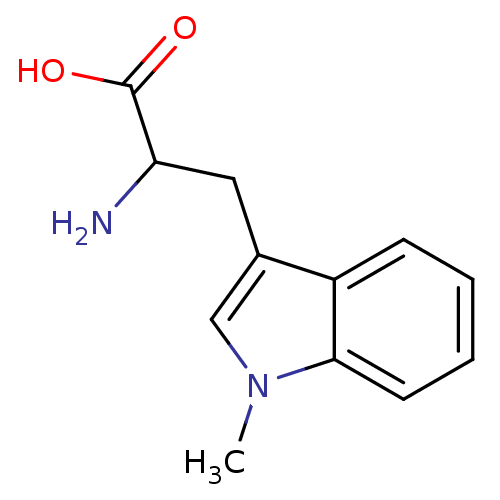 (1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24813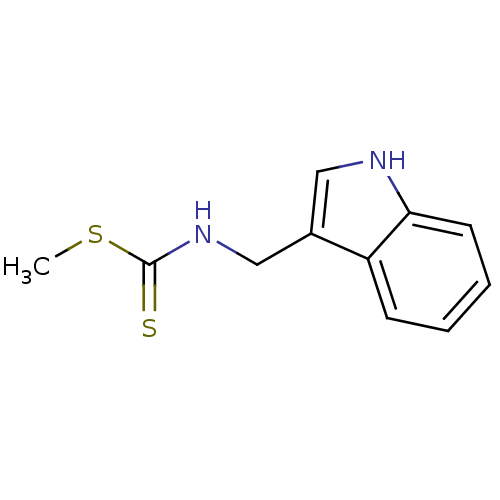 (Brassinin, 1 | N-(1H-indol-3-ylmethyl)(methylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429162 (CHEMBL2336694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429160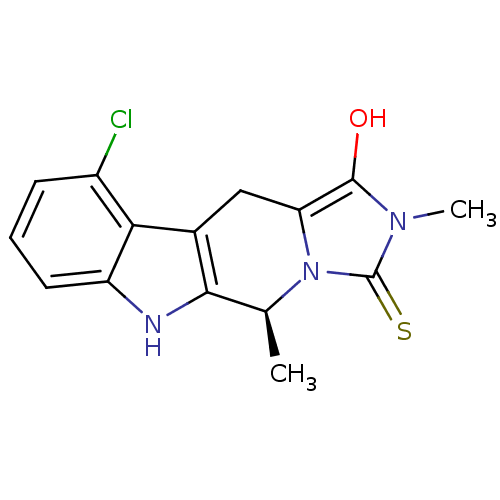 (CHEMBL2336696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429165 (CHEMBL2336706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429166 (CHEMBL2336705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429154 (CHEMBL2336702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429155 (CHEMBL2336701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429164 (CHEMBL2336692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429163 (CHEMBL2336693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM36371 (5-(1H-indol-3-ylmethyl)-3-methyl-2-thioxo-4-Imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429153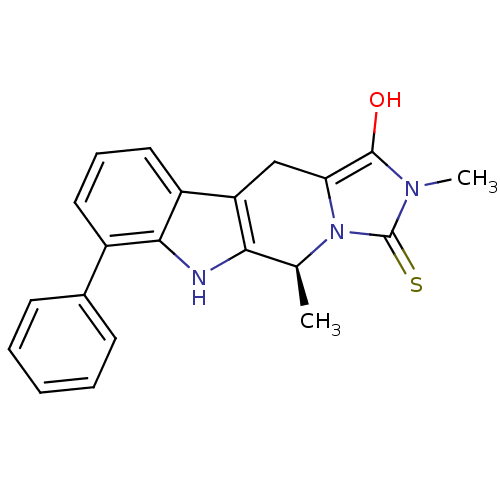 (CHEMBL2336703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429167 (CHEMBL2336704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429158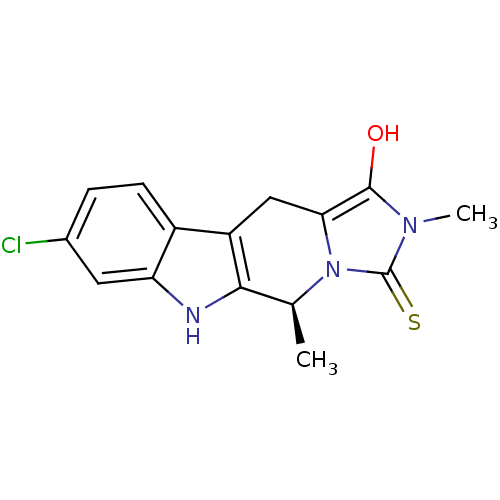 (CHEMBL2336698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429156 (CHEMBL2336700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429161 (CHEMBL2336695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429159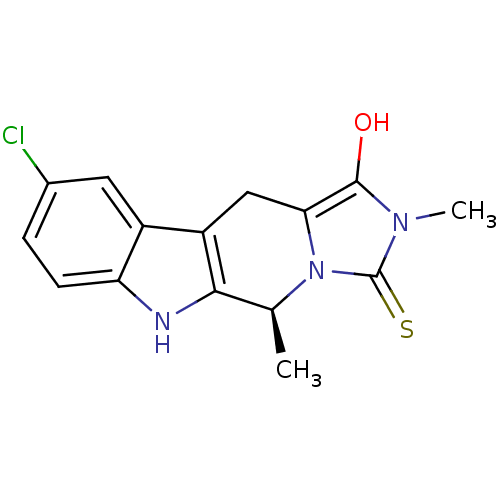 (CHEMBL2336697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429157 (CHEMBL2336699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50429152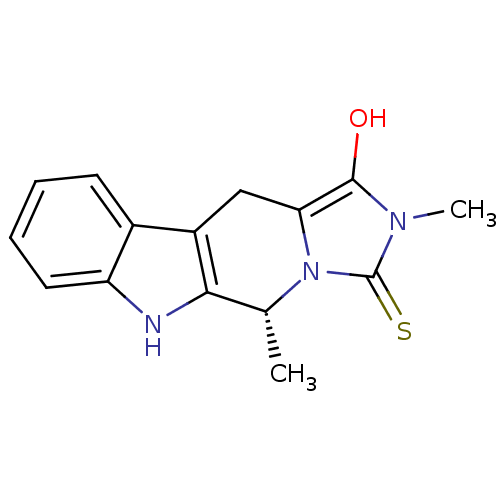 (CHEMBL2336691) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis | Bioorg Med Chem 21: 1159-65 (2013) Article DOI: 10.1016/j.bmc.2012.12.028 BindingDB Entry DOI: 10.7270/Q2RX9DDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||