

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50444531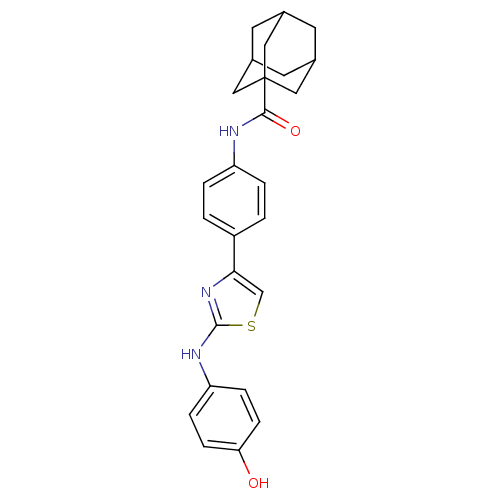 (CHEMBL3099680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipoxygenase (unknown origin) expressed in Escherichia coli BL21(DE3) using arachidonic acid as substrate incubated for 1... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444531 (CHEMBL3099680) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444538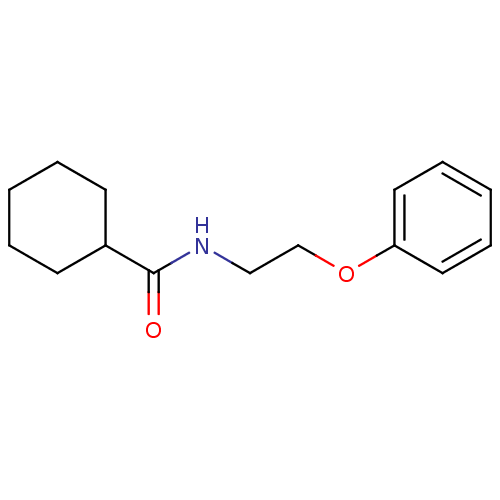 (CHEMBL1313977) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50444532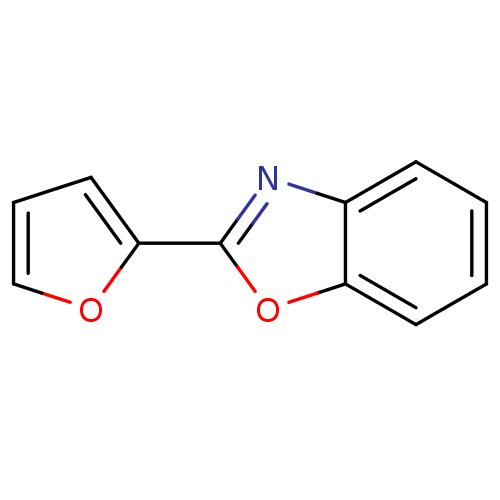 (CHEMBL3099679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipoxygenase (unknown origin) expressed in Escherichia coli BL21(DE3) using arachidonic acid as substrate incubated for 1... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50444534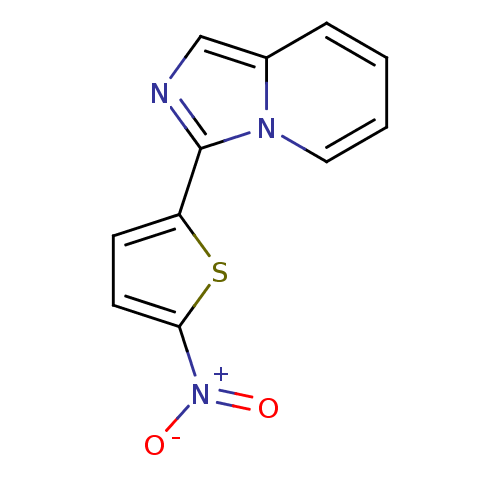 (CHEMBL3099594) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipoxygenase (unknown origin) expressed in Escherichia coli BL21(DE3) using arachidonic acid as substrate incubated for 1... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444537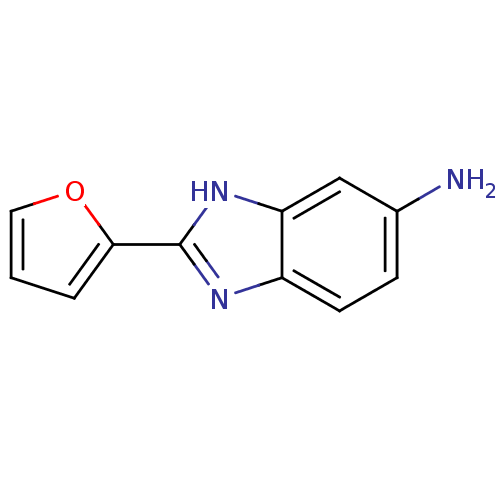 (CHEMBL3099590) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50444535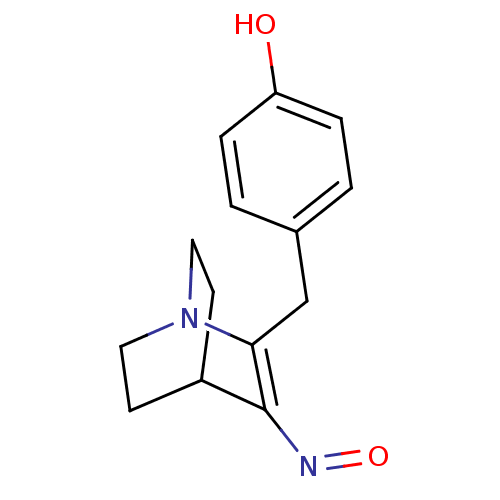 (CHEMBL3099592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipoxygenase (unknown origin) expressed in Escherichia coli BL21(DE3) using arachidonic acid as substrate incubated for 1... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444539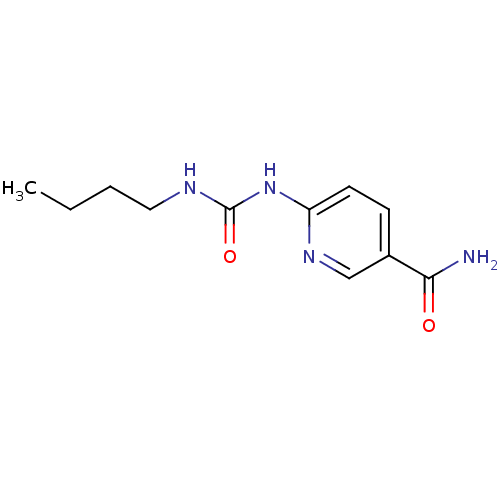 (CHEMBL3099593) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50444537 (CHEMBL3099590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipoxygenase (unknown origin) expressed in Escherichia coli BL21(DE3) using arachidonic acid as substrate incubated for 1... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444540 (CHEMBL1594316) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50444533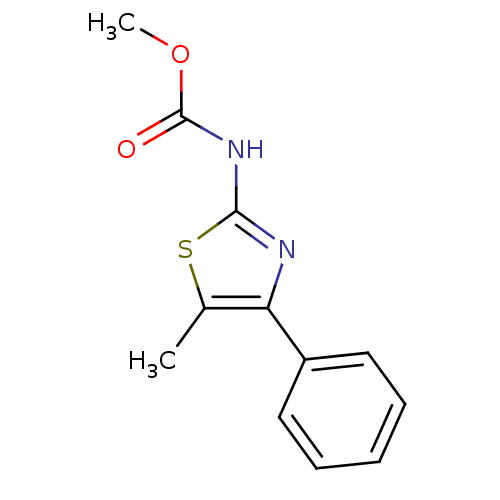 (CHEMBL3099678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipoxygenase (unknown origin) expressed in Escherichia coli BL21(DE3) using arachidonic acid as substrate incubated for 1... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444536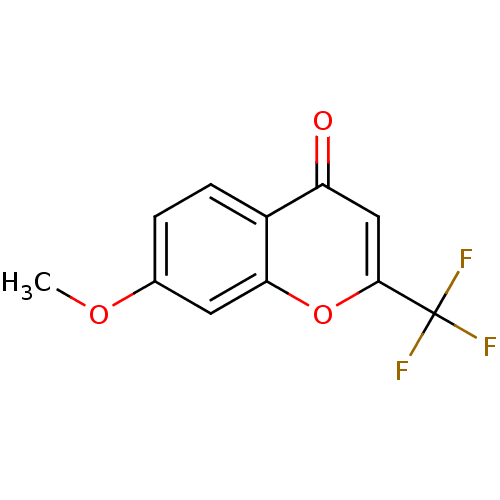 (CHEMBL3099591) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50078827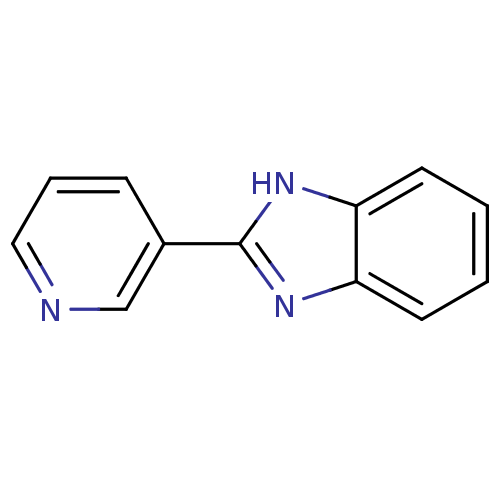 (2-(pyridin-3-yl)-1H-benzo[d]imidazole | 2-Pyridin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444535 (CHEMBL3099592) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444532 (CHEMBL3099679) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50180740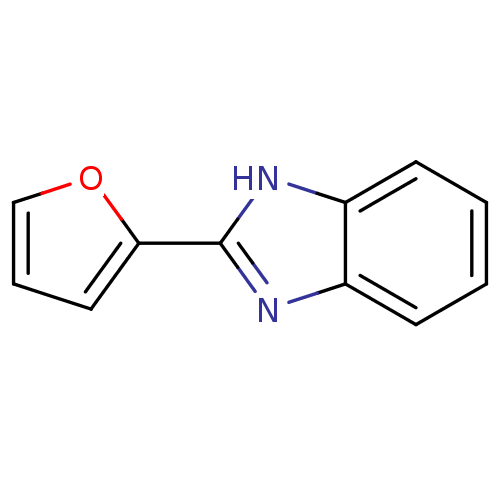 (2-(furan-2-yl)-1H-benzo[d]imidazole | CHEMBL201094...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50444536 (CHEMBL3099591) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipoxygenase (unknown origin) expressed in Escherichia coli BL21(DE3) using arachidonic acid as substrate incubated for 1... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444533 (CHEMBL3099678) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||