

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50099601 (CHEMBL3343930) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099603 (CHEMBL3343932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099597 (CHEMBL3343926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099602 (CHEMBL3343931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099598 (CHEMBL3343927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099599 (CHEMBL3343928) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099591 (CHEMBL3343922) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099598 (CHEMBL3343927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099591 (CHEMBL3343922) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099570 (CHEMBL3343885) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099596 (CHEMBL3343882) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099602 (CHEMBL3343931) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099598 (CHEMBL3343927) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099601 (CHEMBL3343930) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099591 (CHEMBL3343922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099570 (CHEMBL3343885) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099570 (CHEMBL3343885) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099602 (CHEMBL3343931) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099596 (CHEMBL3343882) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099569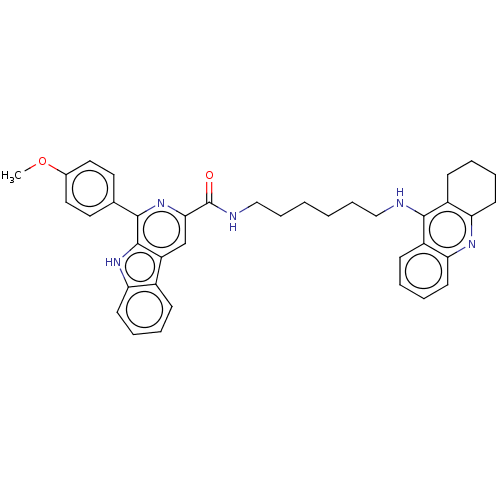 (CHEMBL3343925) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099596 (CHEMBL3343882) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099590 (CHEMBL3343920) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099603 (CHEMBL3343932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099601 (CHEMBL3343930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099592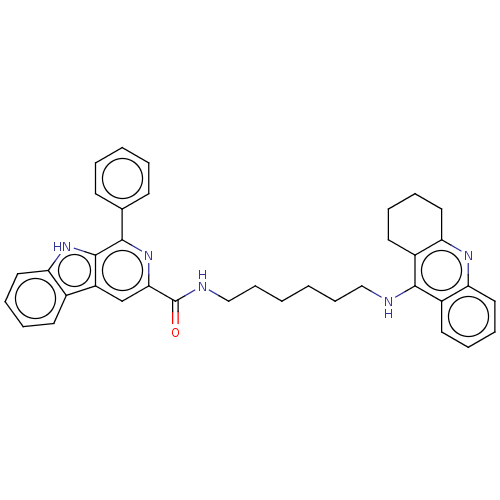 (CHEMBL3343923) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099592 (CHEMBL3343923) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099597 (CHEMBL3343926) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099569 (CHEMBL3343925) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099603 (CHEMBL3343932) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099590 (CHEMBL3343920) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099600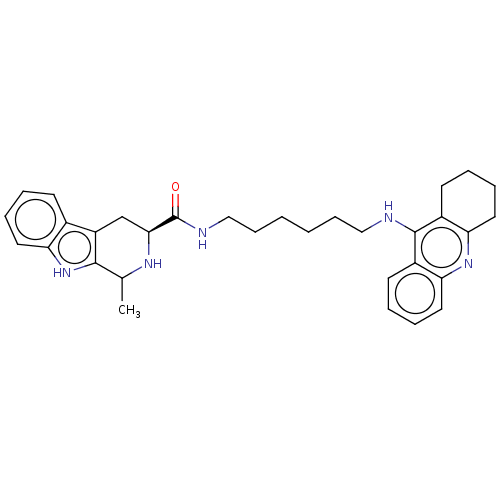 (CHEMBL3343929) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099595 (CHEMBL3343884) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099592 (CHEMBL3343923) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099597 (CHEMBL3343926) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099600 (CHEMBL3343929) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099594 (CHEMBL3343924) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099594 (CHEMBL3343924) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099600 (CHEMBL3343929) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099590 (CHEMBL3343920) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099595 (CHEMBL3343884) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099594 (CHEMBL3343924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099595 (CHEMBL3343884) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099569 (CHEMBL3343925) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 329 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50099593 (CHEMBL3343921) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 364 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine chloride as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099599 (CHEMBL3343928) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 467 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50099589 (CHEMBL3343883) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099599 (CHEMBL3343928) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 6 mins by spectrophotometric method | Bioorg Med Chem 22: 6089-104 (2014) Article DOI: 10.1016/j.bmc.2014.08.035 BindingDB Entry DOI: 10.7270/Q2M61N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |