

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109760 (US8609708, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50113851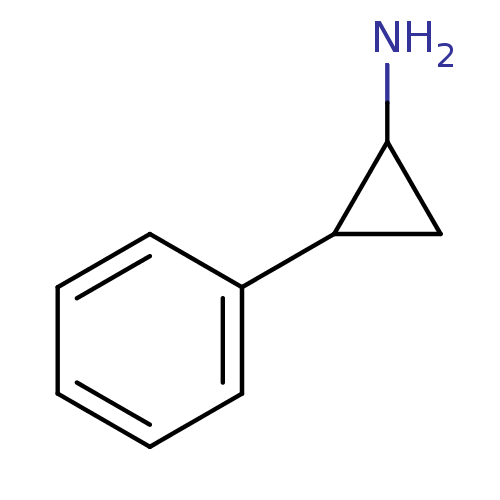 ((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12341 (CHEMBL178681 | CHEMBL359657 | US8609708, 1 | US860...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12345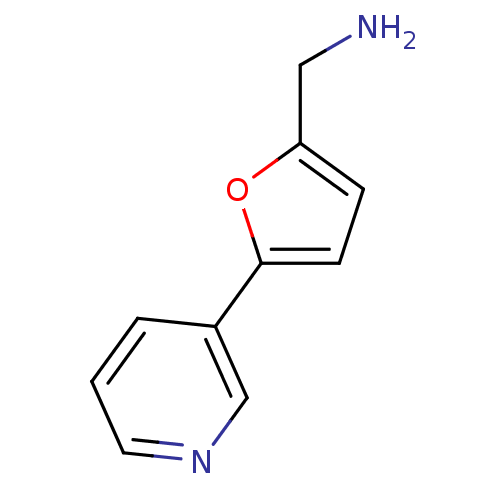 (CHEMBL178090 | US8609708, 2 | US8609708, 47 | [5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB US Patent | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109753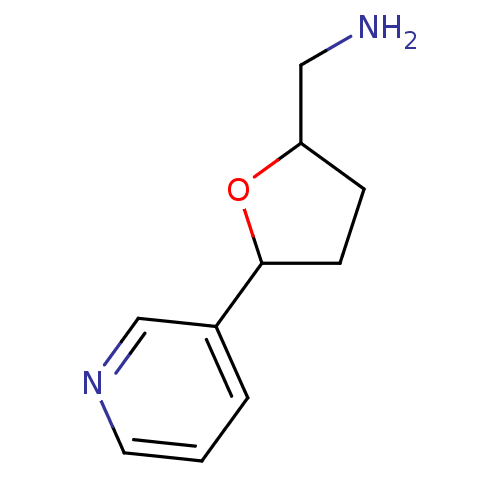 (US8609708, 29 | US8609708, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109748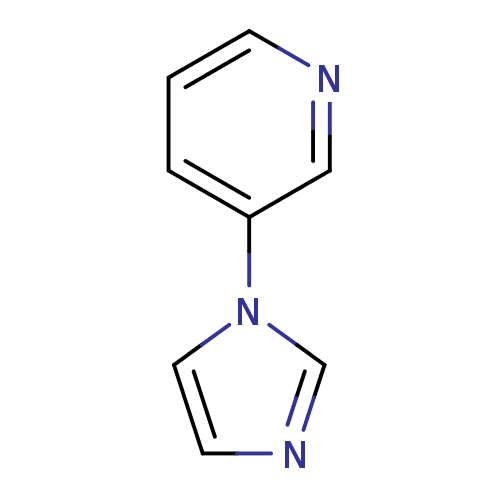 (CHEMBL179477 | US8609708, 22 | US8609708, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109754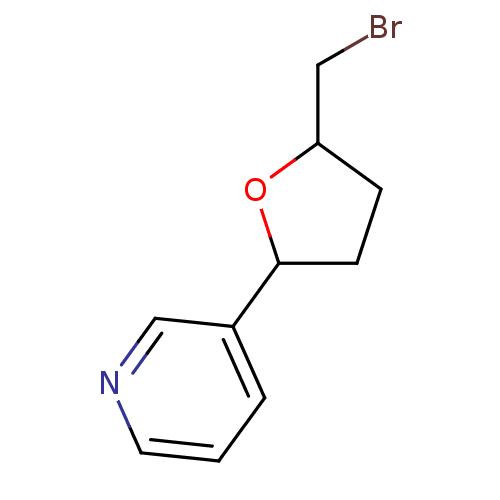 (US8609708, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12348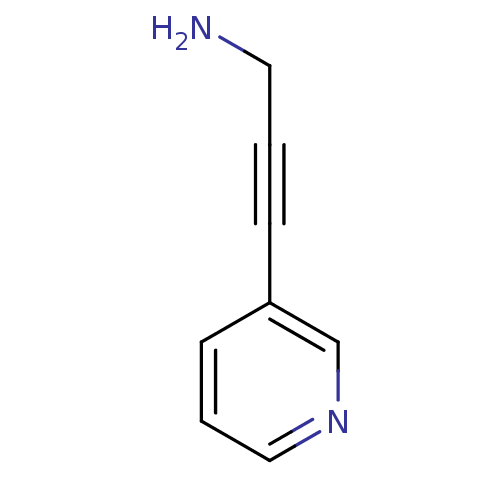 (3-(pyridin-3-yl)prop-2-yn-1-amine | CHEMBL360541 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 514 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12351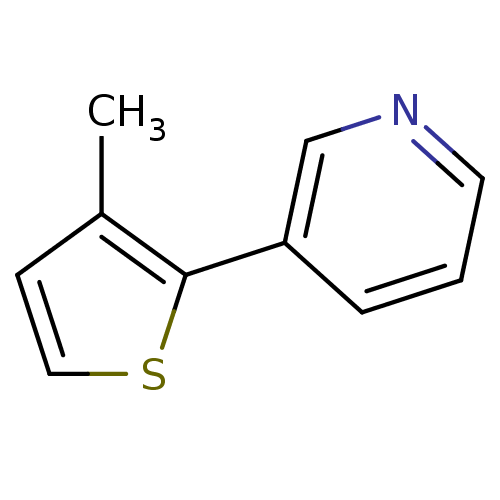 (3-(3-methylthiophen-2-yl)pyridine | CHEMBL179669 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 622 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12352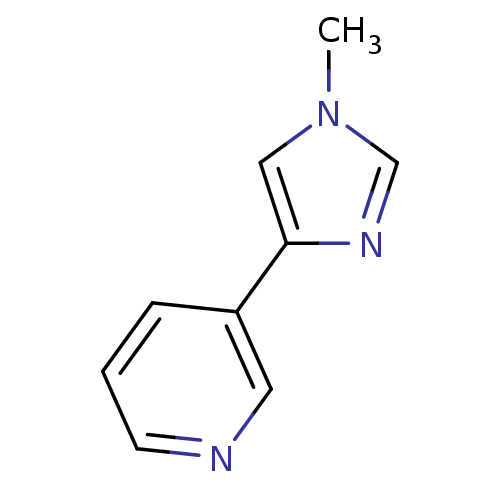 (3-(1-methyl-1H-imidazol-4-yl)pyridine | CHEMBL3609...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 748 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50113851 ((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50113851 ((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109747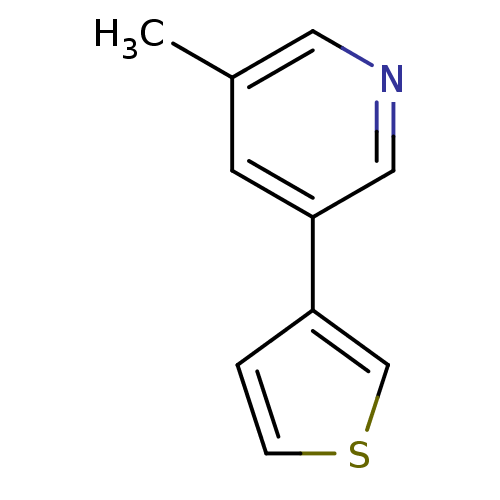 (US8609708, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109748 (CHEMBL179477 | US8609708, 22 | US8609708, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109753 (US8609708, 29 | US8609708, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12343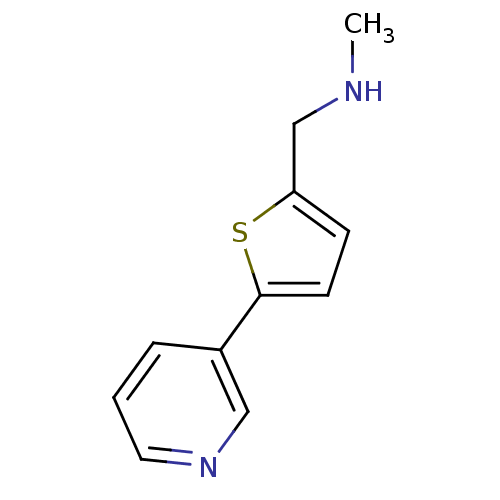 (CHEMBL369285 | US8609708, 9 | methyl({[5-(pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12354 (2-fluoro-5-(3-methylthiophen-2-yl)pyridine | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12355 (3-(thiophen-3-yl)pyridine | CHEMBL361153 | US86097...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158914 (3-(1-Benzyl-1H-imidazol-4-yl)-pyridine | CHEMBL178...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12356 (3-(2-methyl-1H-imidazol-1-yl)pyridine | CHEMBL3688...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12357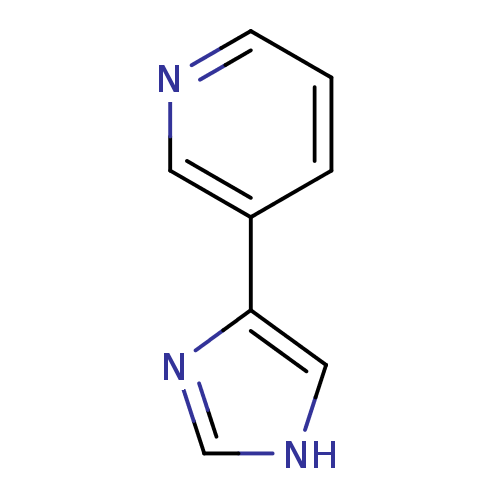 (3-(1H-imidazol-4-yl)pyridine | CHEMBL178516 | JMC5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12357 (3-(1H-imidazol-4-yl)pyridine | CHEMBL178516 | JMC5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM109751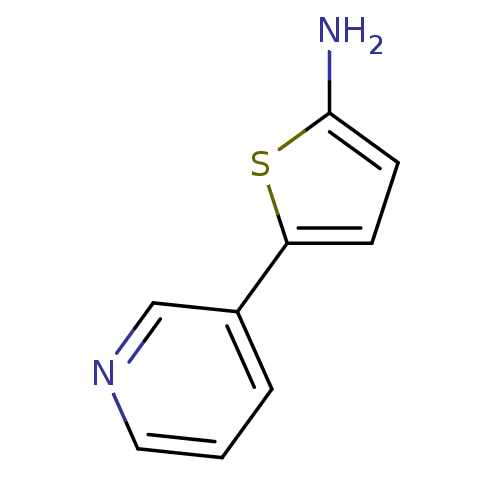 (US8609708, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds, nicotine, nicotine related alkaloids and nicotine metabolites for inhibition of othe... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109749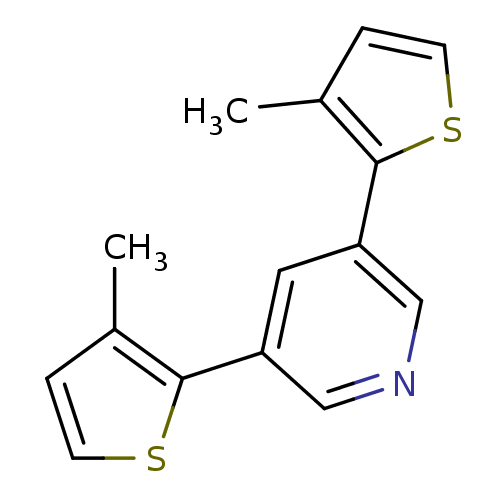 (US8609708, 15 | US8609708,15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12346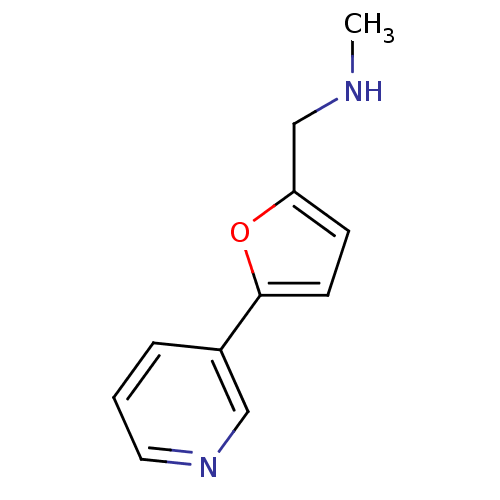 (CHEMBL178938 | US8609708,16 | methyl({[5-(pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB US Patent | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12358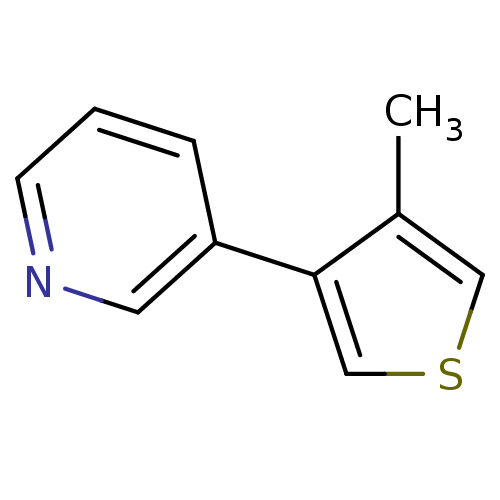 (3-(4-methylthiophen-3-yl)pyridine | CHEMBL179704 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12358 (3-(4-methylthiophen-3-yl)pyridine | CHEMBL179704 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158923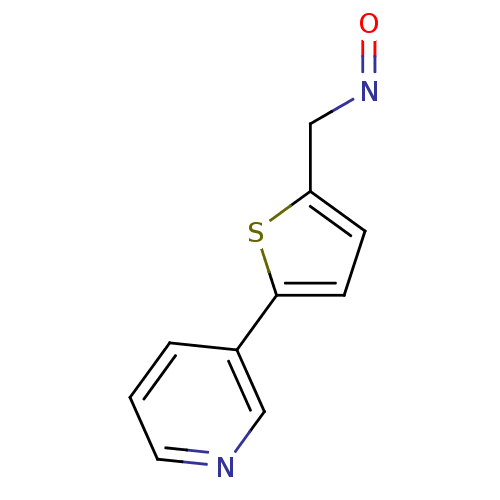 (5-Pyridin-3-yl-thiophene-2-carbaldehyde oxime | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109750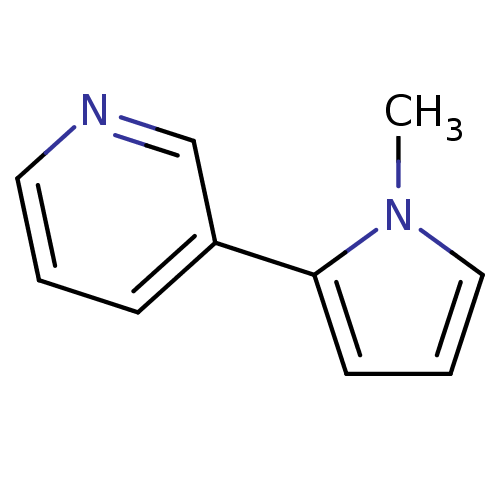 (US8609708, 19 β-Nicotyrine | US8609708, 19 be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158919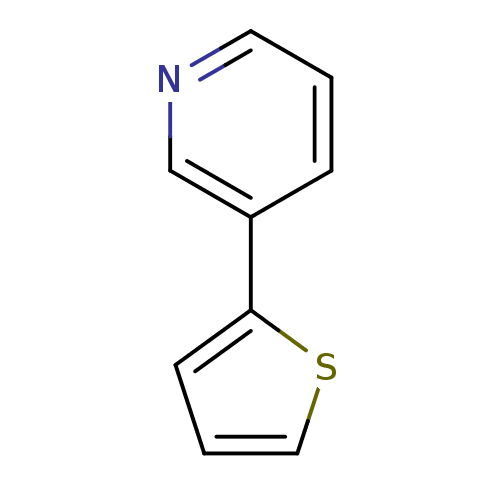 (3-Thiophen-2-yl-pyridine | CHEMBL179618 | US860970...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158928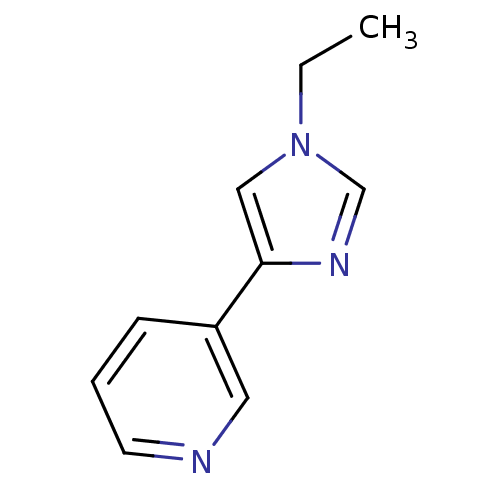 (3-(1-Ethyl-1H-imidazol-4-yl)-pyridine | CHEMBL3613...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109751 (US8609708, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109748 (CHEMBL179477 | US8609708, 22 | US8609708, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM45886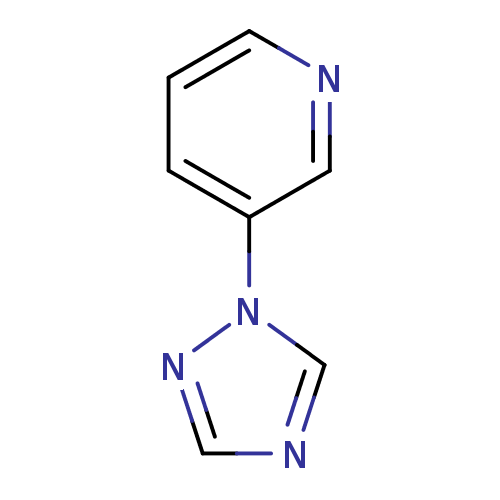 (3-(1,2,4-triazol-1-yl)pyridine | MLS-0091919.0001 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158921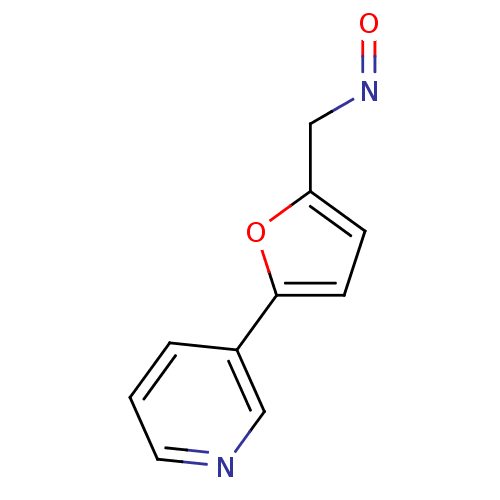 (5-Pyridin-3-yl-furan-2-carbaldehyde oxime | 5-Pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158916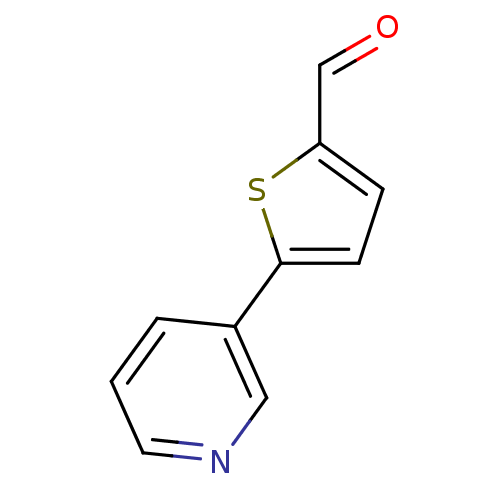 (5-Pyridin-3-yl-thiophene-2-carbaldehyde | CHEMBL17...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109753 (US8609708, 29 | US8609708, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12349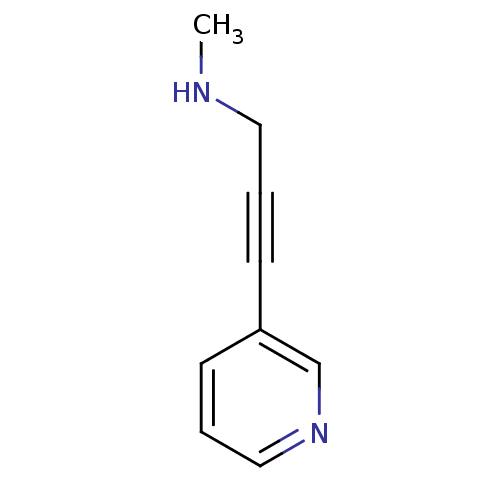 (CHEMBL149808 | US8609708, 26 | methyl[3-(pyridin-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 5.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109752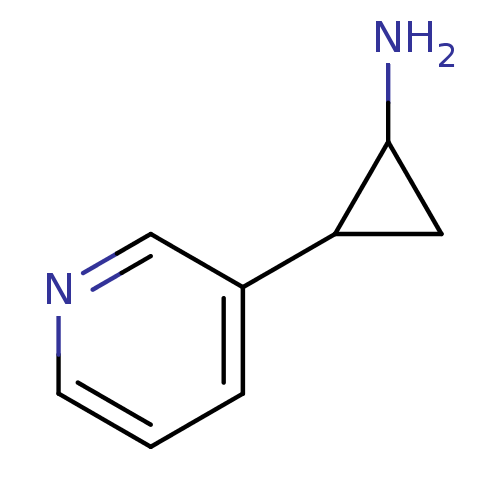 (US8609708, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 5.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158913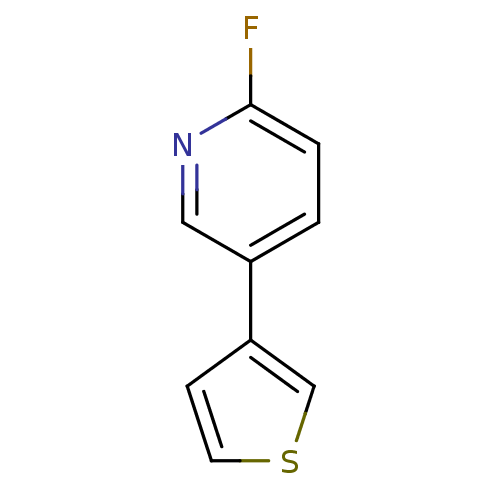 (2-Fluoro-5-thiophen-3-yl-pyridine | CHEMBL179005 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 5.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109753 (US8609708, 29 | US8609708, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109753 (US8609708, 29 | US8609708, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM12351 (3-(3-methylthiophen-2-yl)pyridine | CHEMBL179669 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds, nicotine, nicotine related alkaloids and nicotine metabolites for inhibition of othe... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158918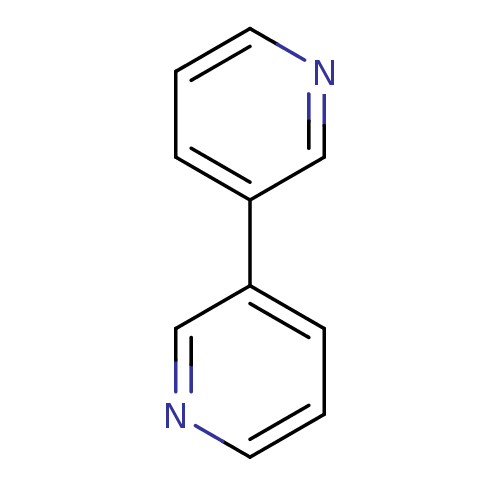 (CHEMBL179763 | US8609708, 30 | [3,3'']Bipyridinyl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158918 (CHEMBL179763 | US8609708, 30 | [3,3'']Bipyridinyl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12341 (CHEMBL178681 | CHEMBL359657 | US8609708, 1 | US860...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 7.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109754 (US8609708, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM109754 (US8609708, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158919 (3-Thiophen-2-yl-pyridine | CHEMBL179618 | US860970...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50158919 (3-Thiophen-2-yl-pyridine | CHEMBL179618 | US860970...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute US Patent | Assay Description The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ... | US Patent US8609708 (2013) BindingDB Entry DOI: 10.7270/Q2PN9481 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 141 total ) | Next | Last >> |