

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298445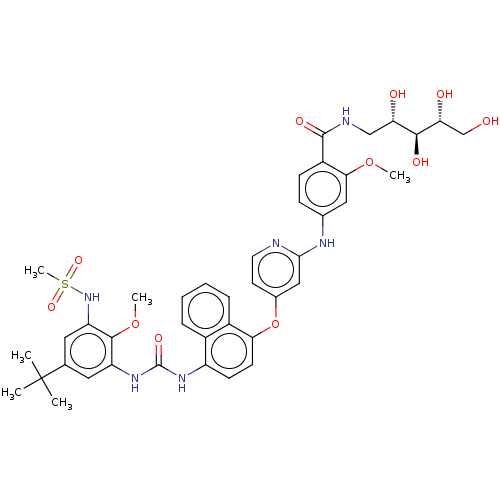 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM337707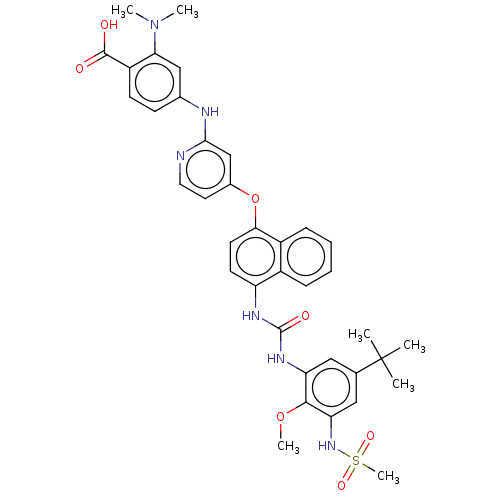 (US10392346, Example 17(am) | US10941115, Example 1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298449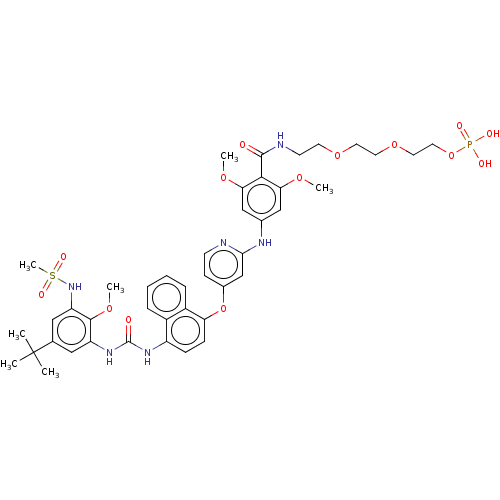 (2-(2-(2-(4-((4-((4-(3-(5-(tert-butyl)-2-methoxy-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM255476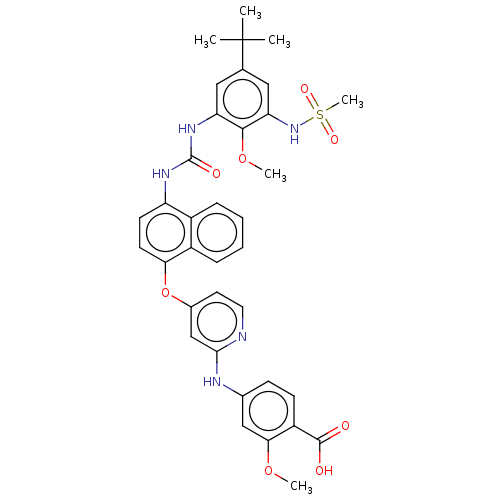 (US10125100, Example 1 | US10392346, Example 1 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298426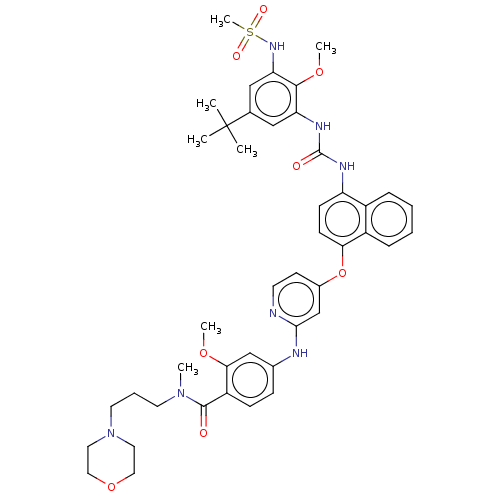 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298439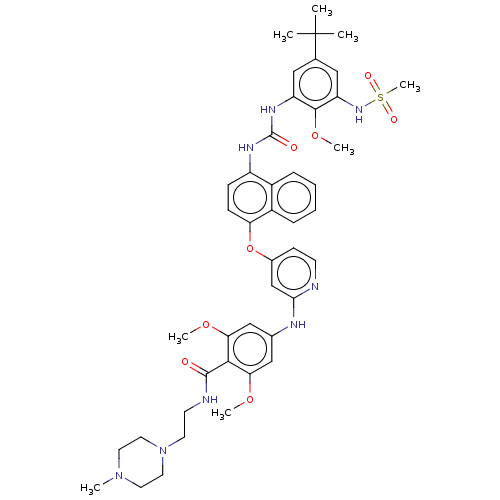 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298442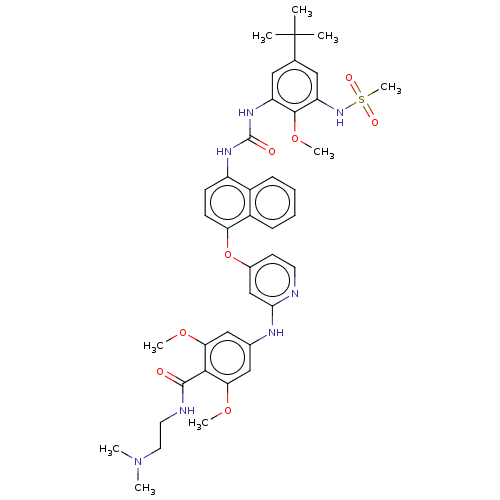 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298436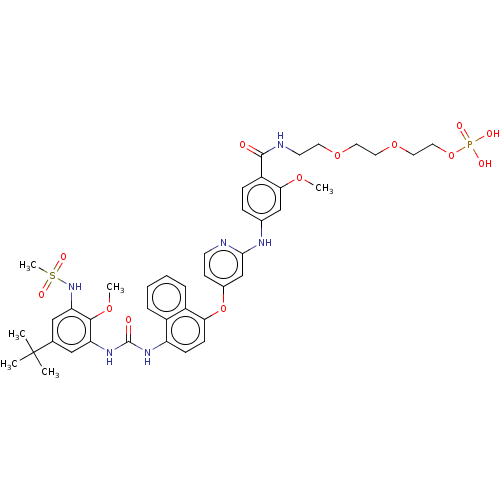 (2-(2-(2-(4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298449 (2-(2-(2-(4-((4-((4-(3-(5-(tert-butyl)-2-methoxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298436 (2-(2-(2-(4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298441 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298425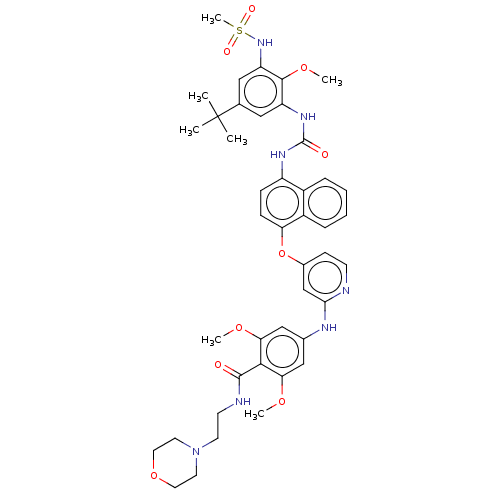 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298392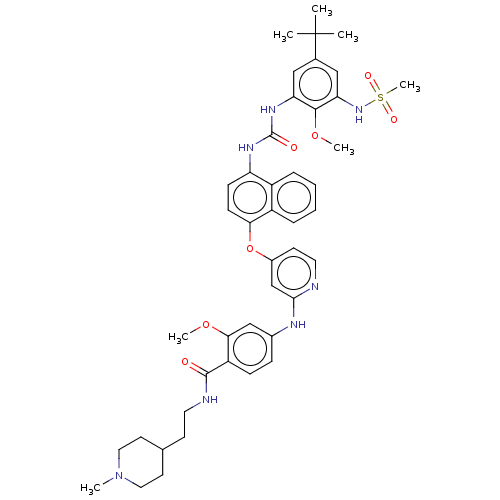 (4-[[4-[[4-[[5-tert-Butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM255476 (US10125100, Example 1 | US10392346, Example 1 | US...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298446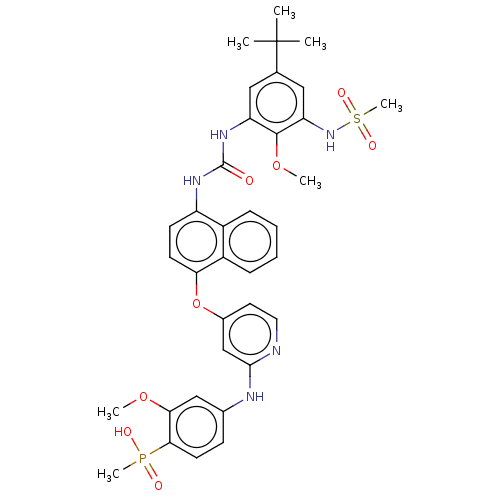 ((4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methyls...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298430 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298431 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298444 (4-((4-((4-(3-(5-(tert-Butyl)-3-(2-hydroxyacetamido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298429 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM255476 (US10125100, Example 1 | US10392346, Example 1 | US...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298449 (2-(2-(2-(4-((4-((4-(3-(5-(tert-butyl)-2-methoxy-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298438 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM411450 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298438 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298404 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM411487 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298446 ((4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methyls...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298442 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298441 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298407 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298392 (4-[[4-[[4-[[5-tert-Butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298446 ((4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methyls...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298450 (4-((4-((4-(3-(5-(tert-Butyl)-3-(N-(2-hydroxyethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298375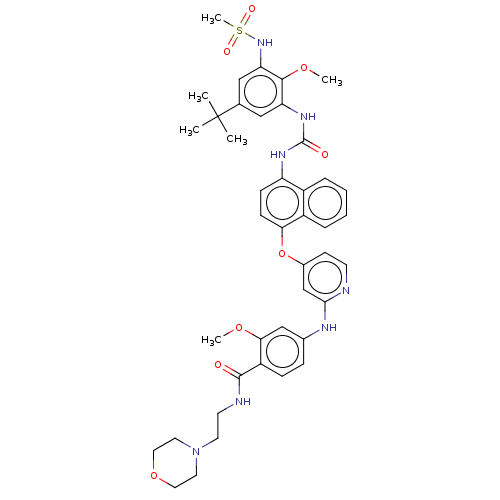 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298375 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298435 (US10125100, Example 17(cp) | US10392346, Example 1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298410 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298421 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM411488 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298396 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298445 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298410 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298404 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298382 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298436 (2-(2-(2-(4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298394 (4-[[4-[[4-[[5-tert-Butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298390 (1-[5-tert-Butyl-3-(methanesulfonamido)-2-methoxy-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298421 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298407 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 187 total ) | Next | Last >> |